Figure 7.
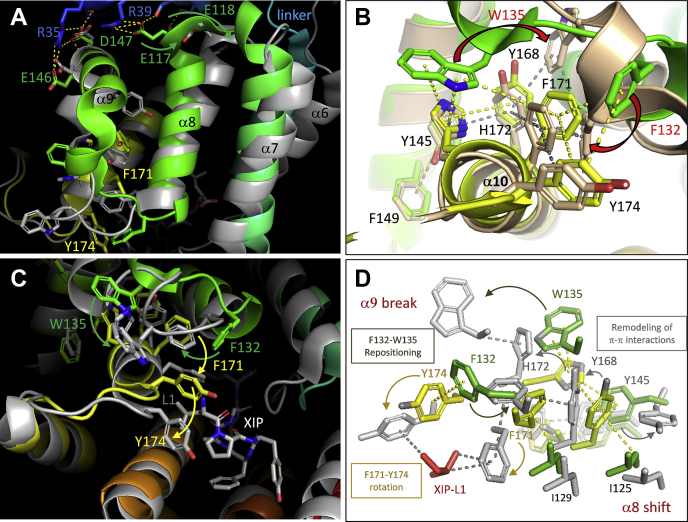
Remodeling of a network of aromatic–aromatic interactions in ComR activation.A, XIP-driven reorganization of TPR-1 (α6–α7) and TPR-2 (α8–α9). Apo-ComRSth (colored by spectrum; PDB ID 5JUF (33)) and ComRSth·XIPSth complex (gray; PDB ID 5JUB (35)) are superimposed. At the top, the helix-α8 reorientation is responsible of the break of R39-R51/E117-E118 salt bridges, which contributes to helix−α9 destabilization needed for the HTH domain release (break of R35/E146-D147 salt bridges). At the bottom, the remodeling of loop (α8–α9) and the reorientation of aromatic residues F171-Y174 are observed. B, F171-Y174 aromatic–aromatic interactions in apo-ComR. The major form (colored by spectrum; PDB ID 5JUF (33)) and a minor form (beige; Chain A, PDB ID 6QER (34)) of apo-ComRSth are superimposed. The network of observed aromatic–aromatic interactions is modified by the repositioning of F132 and W135 from loop α8 to α9 (red arrows). Key residues from helix α9 (Y145 and F149) and helix α10 (Y168, F171, H172 and Y174) are indicated. C, XIP-driven reorganization of F171-Y174 aromatic–aromatic interactions. Apo-ComRSth (colored by spectrum; PDB ID 5JUF (33)) and ComRSth·XIPSth complex (gray; PDB ID 5JUB (35)) are superimposed. The rotation of the lateral chains of F171-Y174 in interaction with XIP-L1 and the repositioning of F132 and W135 from loop α8 to α9 are highlighted. D, detailed view of XIP-driven reorganization of aromatic lateral chains surrounding F171-Y174. Apo-ComRSth (colored by spectrum; PDB ID 5JUF (33)) and ComRSth·XIPSth complex (gray; PDB ID 5JUB (35)) are superimposed. The reorientation/repositioning of shown residues participates to helix-α8 shift and helix-α9 break required for HTH domain release. Interactions were mapped using Arpeggio (http://biosig.unimelb.edu.au/arpeggioweb/) (52).