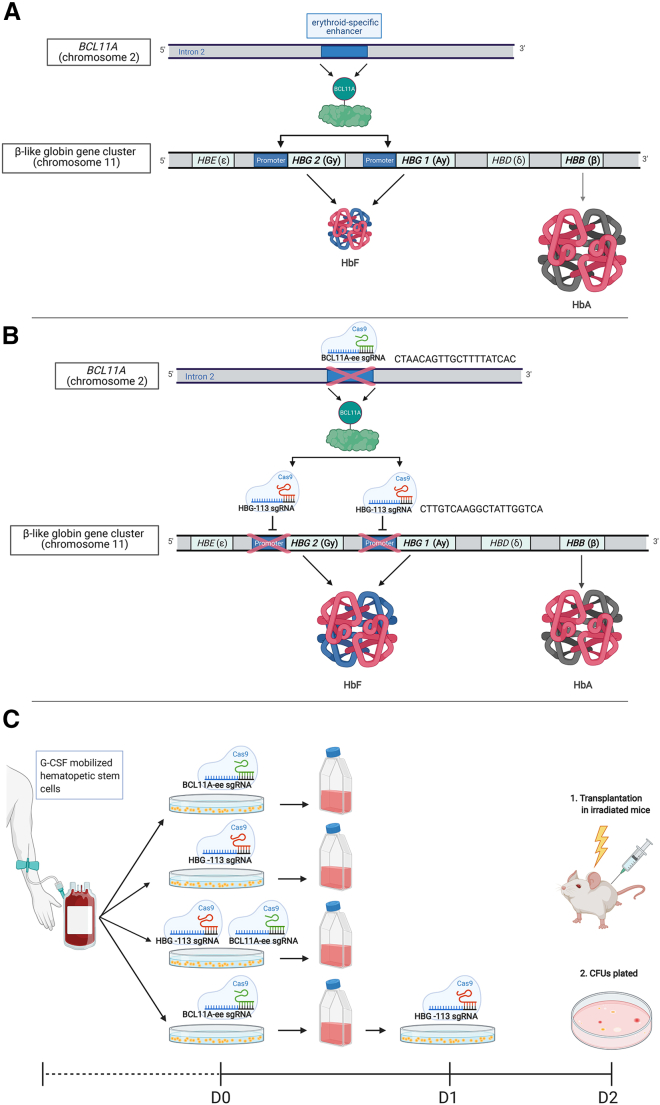
Figure 1.
Outline of rationale and methodology of CRISPR/Cas9 dual-editing strategy
(A) The BCL11A/HBG axis under physiological conditions dictates the switch from fetal hemoglobin (HbF) to adult hemoglobin (HbA) production in infancy. BCL11A transcription factor binds to the promoter regions of HBG, repressing HbF production in favor of HbA. Circulating hemoglobin in most individuals is composed almost entirely of HbA with negligible amounts of HbF. (B) Following CRISPR/Cas9 disruption of the BCL11A erythroid enhancer (BCL11A-ee), there is reduced production of the BCL11A transcription factor in erythroid progenitor cells. Disruption of the HBG promoter (HBG-113) interferes with binding of residual BCL11A protein. These changes lead to disruption of the BCL11A/HBG axis, allowing reversal of the hemoglobin switch and increased HbF production. (C) Outline of experimental protocol.