Abstract
Background
The dynamic monitoring of perioperative carcinoembryonic antigen (CEA) is recommended by current colorectal cancer (CRC) guidelines, while the benefits of additional measurements of carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 125 (CA125) have remained controversial.
Methods
This retrospective longitudinal cohort included 3539 CRC patients who underwent curative resection. Distinct trajectory groups were identified by the latent class growth mixed model. Patients were grouped into subgroups jointly by CEA, CA19-9, and CA125 according to preoperative levels and longitudinal trajectories, respectively. The end points were overall survival (OS) and recurrence-free survival (RFS).
Findings
Three distinct trajectory groups were characterized for serum CEA, CA19-9, and CA125: low-stable, early-rising, and later-rising. Jointly, patients were grouped into six preoperative (trajectory) joint groups. Compared with the three-low group, the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) associated with death were 1.87 (1.29-2.70), 3.82 (2.37-6.17), 1.87 (0.97-3.61), 2.81 (1.93-4.11), and 4.99 (2.80-8.86) for the CEA-high, CA19-9-high, CA125-high, two-high, and three-high group, respectively. And compared with the three-stable trajectory group, the corresponding HRs (95% CIs) were 1.59 (1.10-2.30), 1.55 (0.77-3.10), 6.25 (4.02-9.70), 4.05 (2.73-6.02), and 12.40 (5.77-26.70) for the five rising trajectory groups, respectively. Similar associations between joint groups and RFS were observed. Notably, the trajectory joint group still had prognostic significance after adjusting for preoperative levels. The CA19-9-high group (HR: 3.82, 95% CI: 2.37-6.17) was associated with higher risk of death than the two-high group (HR: 2.81, 95% CI: 1.93-4.11). Likewise, for the CA125-rising trajectory group and two-rising trajectory group, the HRs (95% CIs) were 6.13 (3.75-10.00) and 3.99 (2.63-6.05) for death, and 3.08 (2.07-4.58) and 2.10 (1.52-2.90) for recurrence.
Interpretation
In addition to CEA, the dynamic measurements of CA19-9 and CA125 are recommended to monitor the prognosis of CRC patients.
Funding
National Natural Science Foundation of China [81973147, 82001986, 81960592, 82073569, 81660545].
Keywords: Colorectal cancer, Serum tumor markers, Trajectories, Overall survival, Recurrence-free survival
Abbreviations: CRC, colorectal cancer; CEA, carcinoembryonic antigen; CA19-9, carbohydrate antigen 19-9; CA125, carbohydrate antigen 125; OS, overall survival; RFS, recurrence-free survival; LCGMM, latent class growth mixed model; HR, hazard ratio; CI, confidence interval
Research in context.
Evidence before this study
We searched PubMed for articles published up to Dec 30, 2020, with the terms “colorectal cancer”, “prognosis”, “serum tumor markers”. Carcinoembryonic antigen (CEA) is the most commonly used marker for CRC, and the preoperative and postoperative serum CEA are both associated with the CRC outcomes. In addition to CEA, the prognostic significance of carbohydrate antigen 19-9 (CA19-9) and carbohydrate antigen 125 (CA125) have also been extensively studied in CRC. However, it is unclear whether CA19-9 and CA125 should be considered supplements to CEA for prognostic surveillance of CRC. Besides, most previous studies focused on preoperative or postoperative serum tumor markers in a single or limited number of measurements, with the trajectories not well characterized.
Added value of this study
This retrospective longitudinal cohort identified three distinct trajectory groups for serum carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), and carbohydrate antigen 125 (CA125), characterized by low-stable, early-rising, and later-rising. The longitudinal trajectory was an independent risk factor for prognosis. Compared with preoperative levels, trajectories could further reflect patient's response to surgery and adjuvant therapy, and provided dynamic information. The relative contributions of the preoperative and trajectory joint group of the three markers to predict survival were equivalent to and higher than that of pathological stage, respectively. Compared with patients with no elevated markers, those who had one or more elevated markers had higher risks of death and recurrence. Patients identified in the CA19-9-high and CA125-rising group in the joint analyses had HRs exceeding the two-high (rising) group, providing new insights into the prognostic value of CA19-9 and CA125 in CRC patients with normal CEA.
Implications of all the available evidence
These findings suggest that dynamic measurements of CEA, CA19-9 and CA125 will contribute to the identification of high-risk postoperative CRC patients. In addition to CEA, the dynamic measurements of CA19-9 and CA125 should be recommended to monitor the prognosis of CRC patients.
Alt-text: Unlabelled box
1. Introduction
Colorectal cancer (CRC) is a common malignancy globally [1]. It is vital to design personalized monitoring programs for CRC patients with high risks of recurrence and death. Currently, carcinoembryonic antigen (CEA) is the most commonly used marker for CRC, suggested to be measured preoperatively and every 3-6 months postoperatively by guidelines [2], [3], [4]. In addition to CEA, the prognostic significances of carbohydrate antigen 19-9 (CA19-9) [5,6] and carbohydrate antigen 125 (CA125) [7], [8], [9] have also been extensively studied in CRC.
Current guidelines of CRC recommend routine monitoring of perioperative CEA. However, clinical benefits of additional measurements of other markers, such as CA19-9 and CA125, remain controversial. Although the results of meta-analysis showed significant associations between preoperative CA19-9, CA125 and prognosis, related studies reported heterogeneous results (Supplementary Appendix A). Stiksma et al. suggested that CA19-9 could be used to monitor the disease progression of CRC patients without elevated CEA [10], while the European Group on Tumour Markers guidelines disapproved that the follow-up of CA19-9 provided more prognostic information than CEA [11]. Although studies have reported that CRC patients with simultaneously positive preoperative CEA, CA19-9, and CA125 had the worst prognosis, it is unclear whether CA19-9 and CA125 should be considered supplements to CEA for prognostic surveillance of CRC [12,13].
Previous studies focused on preoperative or postoperative tumor markers in a single or limited number of measurements, ignoring the longitudinal trajectories [14], [15], [16]. Yang et al. analysed multiple tumor markers simultaneously, however, only preoperative levels were involved in their studies [12,13]. Trajectories of tumor markers can reflect dynamic changing patterns during the perioperative period, and provide more information on the relationships of tumor markers with prognosis of CRC patients [17]. We suppose that joint trajectory groups of these tumor markers may better guide the postoperative monitoring.
This longitudinal cohort study aims to identify the trajectories of CEA, CA19-9, and CA125 within three years after surgery, and evaluate the impact of these three tumor markers jointly on CRC outcomes in terms of preoperative levels and longitudinal trajectories.
2. Methods
2.1. Ethics
This multicenter retrospective study was approved by the ethics committee of the Yunnan Cancer Hospital (KY2019141), the ethics committee of the Sixth Affiliated Hospital, Sun Yat-sen University (2021ZSLYEC-051), and the ethics committee of Guangdong Provincial People's Hospital (GDREC2020011H). The requirement for informed consent was waived by the board, owing to the study's retrospective nature. All the patient data in the survey were anonymized.
2.2. Patients
All consecutive CRC patients undergoing curative resection for stage I to III colorectal adenocarcinoma, without neoadjuvant treatment, between January 2011 and February 2019 were retrospectively identified from Yunnan Cancer Hospital, Sixth Affiliated Hospital of Sun Yat-sen University, and Guangdong Provincial People's Hospital in China. Patients with preoperative measurement and three or more measurements within 36 months after surgery were included in the trajectory analysis of CEA, CA19-9, or CA125. Joint analyses were implemented in participants who met the inclusion criteria of the three trajectory analyses simultaneously. The specific inclusion and exclusion process is shown in Figure S1 (Supplementary Appendix B).
2.3. Serum marker determination
Preoperative measurement was defined as the value closest to the time of surgery within four weeks before surgery, and postoperative measurements were repeatedly measured after surgery, with different intervals and times for participants. All measurements were made by chemiluminescence immunoassay using the COBAS 800 e602 immunoassay analyser (Roche Diagnostics, Tokyo, Japan) at Yunnan Cancer Hospital, Alinity immunoassay analyser (Abbott Diagnostics, Chicago, USA) at the Sixth Affiliated Hospital of Sun Yat-sen University, and UniCel DxI 800 immunoassay analyser (Beckman Coulter, USA) at Guangdong Provincial People's Hospital, following World Health Organization standard methods (code 73/601).
2.4. Surveillance protocol and outcome
The surveillance protocol was detailed in previous study [18]. In this study, the follow-up ended on June 30, 2020. The primary endpoint was overall survival (OS). The secondary endpoint was recurrence-free survival (RFS), and recurrence included the local recurrence and distant metastases. The RFS time was defined as the time from surgery to a confirmed recurrence. All recurrent cases were confirmed via histology of biopsy samples or positive imaging.
2.5. Covariates
Covariates included age, sex, surgical approach (open resection or laparoscopic resection), primary site (colon or rectum), tumor differentiation, TNM stage, lymph node yield, mucinous (colloid) type, lymphovascular invasion, perineural invasion, and adjuvant chemotherapy. Preoperative levels of CEA, CA19-9 and CA125 were additionally adjusted in the associations between their trajectories and CRC outcomes.
2.6. Statistical analysis
Due to the high intra-variability of these tumor markers, values of CEA, CA19-9, and CA125 were truncated to ten times the upper limits of their reference intervals [19] to facilitate the fitting of the trajectories. Logarithms of these measurements were used for trajectory fitting due to their non-normality.
A latent class growth mixed model (LCGMM) was used to determine different trajectory patterns of perioperative CEA, CA19-9, and CA125. LCGMM divides heterogeneous populations by estimating latent classes, and fits the individual curve through a linear mixed model [20,21]. We set the longitudinal measurements as linear or nonlinear functions of time (months between each measurement date and the surgery date), expressed as time, the quadratic or cubic term of time, and traversed 2-5 potential groups [17,22]. Participating province and its interaction with time polynomials were also included in our models to account for the pattern differences of regions. The optimal number of groups and the best fitting shape were determined according to the Bayesian information criterion while ensuring that each group had an acceptable proportion of the population and posterior probability (Supplementary Appendix B). LCGMM was implemented with package “lcmm” (version 1.9.2) in R (version 3.6.3). The associations between trajectory groups and CRC outcomes were evaluated by cox proportional hazard models, with age, sex, and preoperative levels adjusted.
The cut-off values for preoperative CEA, CA19-9, and CA125 were set the upper limits of their reference intervals. Patients were assigned into dichotomous preoperative groups (low or high) of each marker based on corresponding cut-off value, and dichotomous trajectory groups (stable or rising) of each marker based on corresponding trajectory patterns. In the joint analysis, comprehensively considering the three markers, preoperative (trajectory) joint groups were formed based on the number of high (rising) markers, and the one-high (rising) group was further subdivided based on the positive marker. Characteristics across different joint groups were compared using Kruskal-Wallis tests for continuous variables, described as median [quartile] and χ2 tests for categorical variables, described as number (%).
Survival curve of each group was drawn using the OS or RFS estimated by Kaplan-Meier, and log-rank test was performed to determine the overall difference between groups. Cox proportional hazard models were used to investigate the associations between the joint groups and outcomes. Three models were used: model 1 with no covariate; model 2 with adjustment for age, sex; model 3 with further adjustment for primary site, surgical approach, tumor differentiation, pathology stage, lymph node yield, mucinous (colloid) type, lymphovascular invasion, perineural invasion, and adjuvant chemotherapy. We further adjusted preoperative markers in the analysis of trajectory joint group. The relative importance of each variable to predict OS and RFS was evaluated using the χ2 proportion test, performed with Harrell's rms R package (version 3.6.3).
2.7. Subgroup analysis
To study potential heterogeneity, we stratified participants by participating province, sex, primary site, surgical approach, pathology stage, tumor differentiation, lymph node yield, and adjuvant chemotherapy, repeated the cox analysis in each subgroup, and included the multiplication term to test the existence of interaction.
All statistical analyses mentioned above were performed using R software (version 3.6.3; http://www.R-project.org).
2.8. Role of the funding source
The funding sources had no role in the study design, data collection, analysis, interpretation, or writing of the manuscript. T.Z., D.Y., and Z.L. had full access to the raw data of the study database. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
3. Results
Of the 3539 patients who underwent surgery for colorectal cancer without preoperative therapy between January 2011 and December 2018 at participating centers, 2160, 2067, and 2061 patients were enrolled in the trajectory analysis of CEA, CA19-9, and CA125, with 17,836 (2.37% truncated), 16,933 (1.38% truncated), and 17,031 (0.08% truncated) measurements, respectively. The measurements number of all three markers ranged from 4-21, with a median of 8. The longitudinal measurement time of trajectory fitting was set to 36 months after surgery, resulting in a median [inter-quartile range] of 12.10 [6.30-22.18], 12.03 [6.27-21.95], 11.67 [6.23-22.10] months for CEA, CA19-9, and CA125.
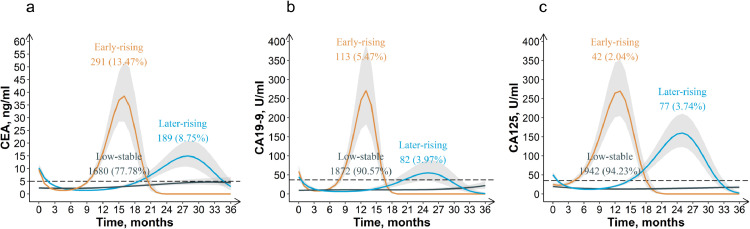
The model fitting results of LCGMM are summarized in Table S1-S3 (Supplementary Appendix B). According to the selection criteria, the cubic curves with three potential groups was optimal for all three tumor markers (Supplementary Appendix B). The trajectories of perioperative tumor markers are shown in Figure 1. Serum CEA, CA19-9, and CA125 all had three similar trajectory groups, named as low-stable, early-rising, and later-rising according to their shapes. In the low-stable group, the three markers remained stable at low levels (CEA: 5 ng/ml; CA19-9: 37 U/ml; CA125: 35 U/ml) during three years after surgery, with no significant increase or decrease. In the early-rising group, these three markers peaked first and then fell to normal in the early postoperative period (approximately 1.5 years after surgery). Similarly, in the later-rising group, the peak appeared in 1.5-3 years after surgery. The proportions of the three trajectory groups were 77.78%, 13.47%, 8.75% for CEA; 90.57%, 5.47%, 3.97% for CA19-9; 94.23%, 2.04%, 3.74% for CA125. The clinicopathological characteristics by trajectory groups are summarized in Table S4-S6 (Supplementary Appendix B).
Figure 1.
Trajectories of perioperative CEA (a), CA19-9 (b) and CA125 (c) in colorectal cancer patients
The associations of CEA, CA19-9, and CA125 trajectory groups with OS and RFS are summarized in Table 1. Compared with the low-stable group, the early-rising and later-rising groups both had higher risks of death (HR and 95% confidence interval (CI): 1.6 (1.2-2.3) and 2.4 (1.6-3.4) for CEA, 2.4 (1.6-3.8) and 2.4 (1.5-3.9) for CA19-9, 14.5 (9.5-22.2) and 4.9 (3.1-7.8) for CA125). Similar associations between trajectory groups and RFS were observed.
Table 1.
Cox proportional hazard regression analysis of the effect of CEA, CA19-9 and CA125 trajectory groups on CRC outcomes
| CEA trajectory groups |
CA19-9 trajectory groups |
CA125 trajectory groups |
||||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| Death | ||||||
| Early-rising vs. Low-stable | 1.6 (1.2-2.3) | 0.006 | 2.4 (1.6-3.8) | < 0.001 | 14.5 (9.5-22.2) | < 0.001 |
| Later-rising vs. Low-stable | 2.4 (1.6-3.4) | < 0.001 | 2.4 (1.5-3.9) | < 0.001 | 4.9 (3.1-7.8) | < 0.001 |
| Recurrence | ||||||
| Early-rising vs. Low-stable | 1.5 (1.1-1.9) | 0.003 | 1.5 (1.1-2.1) | 0.027 | 7.6 (5.2-11.0) | < 0.001 |
| Later-rising vs. Low-stable | 1.6 (1.2-2.1) | 0.003 | 1.4 (1.0-2.1) | 0.105 | 2.1 (1.4-3.1) | < 0.001 |
Note: For CEA trajectory groups, age, sex (female vs. male) and preoperative CEA were adjusted; For CA19-9 trajectory groups, age, sex (female vs. male) and preoperative CA19-9 were adjusted; For CA125 trajectory groups, age, sex (female vs. male) and preoperative CA125 were adjusted.
A total of 1974 patients included in all above three trajectory analyses were used for subsequent joint analyses. This cohort for joint analysis had a median follow-up time of 42.8 (inter-quantile range: 31.6-59.3) months, with 233 (11.8%) deaths and 452 (22.9%) recurrences. The characteristics of 1974 patients by survival outcomes are summarized in Table S7 (Supplementary Appendix B). People who died during follow-up were more likely to have higher age, pathological stage, the proportions of lymphovascular invasion, perineural invasion and adjuvant chemotherapy, as well as lower differentiation degree, the proportions of colon cancer and open resection.
Patients belonging to the early-rising and later-rising trajectory groups of CEA, CA19-9, and CA125 were classified into the trajectory-rising group of each marker. Jointly, patients were classified into six preoperative (trajectory) groups: three-low (three-stable), CEA-high (CEA-rising), CA19-9-high (CA19-9-rising), CA125-high (CA125-rising), two-high (two-rising) and three-high (three-rising). Patients in CEA-high (CEA-rising) group had high (rising) CEA with low (stable) CA19-9 and CA125. Similarly, Patients in CA19-9-high (CA19-9-rising) and CA125-high (CA125-rising) groups had only high (rising) CA19-9 and CA125. Consistency between the preoperative and trajectory joint group is shown in Table 2. The kappa coefficient of the two joint methods was 0.399, showing a non-negligible difference. Patients' characteristics by joint groups are summarized in Table S8-S9 (Supplementary Appendix B). Compared with the three-low (three-stable) group, the group with at least one high (rising) markers had more features associated with poor prognosis. Though the three-rising group had a small number of patients, patients in this group had a lower degree of differentiation and a higher N stage (Table S8-S9 in Supplementary Appendix B). Compared with other groups, the CA125-rising group and three-rising group had relatively higher metastasis rate (Table S10-S11 in Supplementary Appendix B). Though no significant associations were noted between joint groups and site of metastasis, CA125-rising group showed a predisposition to liver metastasis (Table S12-S13 in Supplementary Appendix B).
Table 2.
Consistency between preoperative and trajectory joint groups
| Preoperative joint groups | Trajectory joint groups |
Total | |||||
|---|---|---|---|---|---|---|---|
| Three-stable | CEA-rising | CA19-9-rising | CA125-rising | Two-rising | Three-rising | ||
| Three-low | 898 | 9 | 13 | 18 | 4 | 1 | 943 (47.8) |
| CEA-high | 228 | 229 | 4 | 8 | 16 | 1 | 486 (24.6) |
| CA19-9-high | 65 | 3 | 35 | 5 | 10 | 2 | 120 (6.1) |
| CA125-high | 64 | 3 | 0 | 25 | 0 | 1 | 93 (4.7) |
| Two-high | 106 | 68 | 22 | 12 | 73 | 5 | 286 (14.5) |
| Three-high | 11 | 6 | 9 | 3 | 14 | 3 | 46 (2.3) |
| Total | 1372 (69.5) | 318 (16.1) | 83 (4.2) | 71 (3.6) | 117 (5.9) | 13 (0.7) | 1974 (100) |
Fleiss' Kappa = 0.399, P < 0.001
Note: Data are presented as number or number (%) of patients.
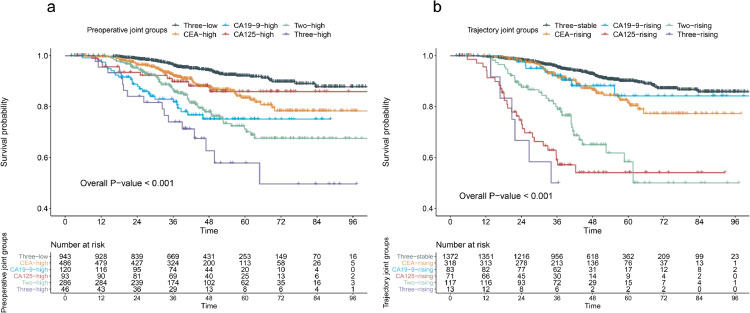
The Kaplan‐Meier curves of OS for the preoperative and trajectory joint groups are shown in Figure 2. As shown in Figure 2a, the 5-year OS rates are 92.1%, 83.4%, 75.1%, 85.9%, 70.1%, 57.8% for three-low, CEA-high, CA19-9-high, CA125-high, two-high, and three-high group, respectively. As shown in Figure 2b, the 5-year OS rates are 90.1%, 81.5%, 84.2%, 54.0%, 58.3%, 26.7% for three-stable, CEA-rising, CA19-9-rising, CA125-rising, two-rising, and three-rising group, respectively. The Kaplan‐Meier curves of RFS are shown in Figure S2 (Supplementary Appendix B). The 5-year RFS rates were 82.3%, 71.7%, 63.7%, 78.1%, 62.2%, 46.2% for the preoperative joint groups (Figure S2a), and 78.6%, 70.4%, 84.1%, 49.3%, 52.5%, 32.3% for the trajectory joint groups (Figure S2b). The overall P (log-rank test) were all < 0.001.
Figure 2.
Kaplan‐Meier curves of overall survival for the preoperative joint groups (a) and trajectory joint groups (b) of CEA, CA19-9 and CA125 in colorectal cancer patients Overall P values were calculated using log-rank test of the six preoperative and trajectory joint groups
The associations of joint groups with outcomes are shown in Table 3. In preoperative joint analysis, compared with the three-low group, the HRs (95%CIs) for death were 1.87 (1.29-2.70), 3.82 (2.37-6.17), 1.87 (0.97-3.61), 2.81 (1.93-4.11), 4.99 (2.80-8.86) for the CEA-high, CA19-9-high, CA125-high, two-high, and three-high group, respectively. Meanwhile, the corresponding HRs (95%CIs) for recurrence were 1.63 (1.28-2.07), 2.19 (1.53-3.14), 1.47 (0.92-2.37), 2.24 (1.72-2.91), 3.50 (2.21-5.53). In trajectory joint analysis, compared with the three-stable group, the HRs (95%CIs) for death were 1.59 (1.10-2.30), 1.55 (0.77-3.10), 6.25 (4.02-9.70), 4.05 (2.73-6.02), 12.40 (5.77-26.70) for the CEA-rising, CA19-9-rising, CA125-rising, two-rising, and three-rising group, respectively. Meanwhile, the corresponding HRs (95%CIs) for recurrence were 1.28 (1.00-1.64), 0.86 (0.49-1.50), 3.32 (2.29-4.80), 2.28 (1.68-3.11), 5.64 (2.74-11.60). The trajectory joint group still had significant prognostic significance after adjusting for preoperative CEA, CA19-9 and CA125. The results with different covariates adjusted are shown in Table S14-S17 (Supplementary Appendix B). The associations were robust across different models. The relative variable importance analysis showed that the most important variables were trajectory joint group, AJCC 8th ed. stage and preoperative joint group. The prognostic values of the preoperative and trajectory joint group of the three markers were equivalent to and higher than that of the pathological stage (Figure S3 in Supplementary Appendix B).
Table 3.
Cox proportional hazard regression analysis of the effect of preoperative and trajectory joint groups on CRC outcomes
| Preoperativejoint model |
Trajectoryjoint model |
Trajectory joint model adjusted preoperative levels |
||||
|---|---|---|---|---|---|---|
| Outcome | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value |
| Death | ||||||
| Three-low (stable) | Reference | Reference | Reference | |||
| CEA-high (rising) | 1.87 (1.29-2.70) | 0.001 | 1.59 (1.10-2.30) | 0.013 | 1.60 (1.09-2.35) | 0.015 |
| CA19-9-high (rising) | 3.82 (2.37-6.17) | < 0.001 | 1.55 (0.77-3.10) | 0.220 | 1.52 (0.76-3.05) | 0.242 |
| CA125-high (rising) | 1.87 (0.97-3.61) | 0.063 | 6.25 (4.02-9.70) | < 0.001 | 6.13 (3.75-10.00) | < 0.001 |
| Two-high (rising) | 2.81 (1.93-4.11) | < 0.001 | 4.05 (2.73-6.02) | < 0.001 | 3.99 (2.63-6.05) | < 0.001 |
| Three-high (rising) | 4.99 (2.80-8.86) | < 0.001 | 12.40 (5.77-26.70) | < 0.001 | 12.00 (5.37-26.60) | < 0.001 |
| Recurrence | ||||||
| Three-low (stable) | Reference | Reference | Reference | |||
| CEA-high (rising) | 1.63 (1.28-2.07) | < 0.001 | 1.28 (1.00-1.64) | 0.051 | 1.24 (0.96-1.61) | 0.099 |
| CA19-9-high (rising) | 2.19 (1.53-3.14) | < 0.001 | 0.86 (0.49-1.50) | 0.589 | 0.83 (0.48-1.47) | 0.530 |
| CA125-high (rising) | 1.47 (0.92-2.37) | 0.110 | 3.32 (2.29-4.80) | < 0.001 | 3.08 (2.07-4.58) | < 0.001 |
| Two-high (rising) | 2.24 (1.72-2.91) | < 0.001 | 2.28 (1.68-3.11) | < 0.001 | 2.10 (1.52-2.90) | < 0.001 |
| Three-high (rising) | 3.50 (2.21-5.53) | < 0.001 | 5.64 (2.74-11.60) | < 0.001 | 5.18 (2.51-10.70) | < 0.001 |
Note: For preoperative and trajectory joint model, age, sex (female vs. male), primary site (rectum vs. colon), surgical approach (open resection vs. laparoscopic resection), tumor differentiation (poor-undifferentiated & moderate vs. well), pathology stage (III→ I), lymph node yield (≥12 vs. <12), mucinous (colloid) type (yes vs. no), lymphovascular invasion (yes vs. no), perineural invasion (yes vs. no), and adjuvant chemotherapy (yes vs. no). For Trajectory joint model adjusted preoperative levels, preoperative CEA, preoperative CA19-9 and preoperative CA125 were further included for adjustment.
According to preoperative patients’ characteristics, exploratory subgroup analyses are shown in Figure S4-S7 (Supplementary Appendix B). This subgroup analyses of OS and RFS found similar results with those for the overall population. For preoperative joint group, there were statistically significant interactions between the surgical approach and the CEA-high and CA125-high group for OS (HR (95% CI) of interaction: 0.39 (0.18-0.85) and 0.24 (0.06-0.90), P (Cox model) = 0.018 and 0.034). For trajectory joint group, there were statistically significant interactions between the surgical approach and the CA125-rising group for OS (HR (95% CI) of interaction: 0.37 (0.15-0.87), P (Cox model) = 0.023), between the tumor differentiation and the two-rising group for OS and RFS (HR (95% CI) of interaction: 2.91 (1.47-5.73) and 1.64 (1.00-2.67), P (Cox model) = 0.002 and 0.048), between the primary site and the three-rising group for OS and RFS (HR (95% CI) of interaction: 14.13 (2.60-76.75) and 6.49 (1.51-27.94), P (Cox model) = 0.002 and 0.012), between the AJCC 8th ed. stage and the three-rising group for OS (HR (95% CI) of interaction: 0.39 (0.17-0.91), P (Cox model) = 0.030).
4. Discussion
In the current study, we identified trajectories of CEA, CA19-9 and CA125 within three years after surgery, and evaluated their impact jointly on CRC outcomes. CEA, CA19-9, and CA125 all had three longitudinal patterns, characterized by low-stable, early-rising, and later-rising. The rising groups were associated with increased risks of recurrence and death than the stable group. And the joint groups’ associations with OS and RFS were significant, independent of existing prognostic factors. Consistency test showed that there was a non-negligible difference between the preoperative and the trajectory joint group, and the trajectory joint group still had prognostic significance after adjusting for preoperative levels of the three markers. The trajectory joint group was the most important variable to predict survival. Compared with patients with no elevated markers, those who had one or more elevated markers had higher risks of poor prognosis. Patients identified in the CA19-9-high and CA125-rising group were high-risk patients, with HRs exceeding the two-high (rising) group. To our knowledge, this is the first longitudinal cohort study exploring the relationships between trajectory groups of multiple tumor markers and outcomes.
In the trajectory analysis, ascending levels of tumor markers indicated poor prognosis, which is consistent with other studies [23,24]. It has been reported that CEA generally dropped to normal within 4-6 weeks after resection in CRC patients [25], [26], [27], and a continued CEA rising after surgery often implied incomplete resection and occult metastasis, which indicated recurrence [25,26]. Previous studies on the dynamic patterns of CEA in CRC also found a rapid elevation group and a slow elevation group, which supports our conclusion to some extent [28]. It is worth noting that the trajectory was a comprehensive reflection of all aspects of the tumor, including response to surgery and adjuvant therapy, which may account for the appearance of a peak in the rising group. After multivariate adjustment, the trajectory grouping of each marker was still significant, indicating that it was an independent predictor of recurrence and death of CRC. Therefore, changes in serial levels of tumor markers are important in the postoperative surveillance of CRC [29], and close attention should be given to patients whose tumor markers show a rising trend.
Multicenter, large-sample longitudinal data were used to illustrate the significance of considering these three markers synthetically. Both the preoperative and the trajectory joint analysis pointed to a positive correlation between the number of elevated markers and the outcomes of recurrence and death, supported by the study of You et al [30]. Both preoperative CEA and CA19-9 were independent risk predictors, with a higher risk of single elevation of CA19-9 than CEA. This is in line with previous studies that endorsed preoperative CA19-9, an additional method to monitor CRC progression in patients with normal CEA [31], [32], [33]. However, during longitudinal follow-up, our results showed that there was insufficient evidence to support the prognostic benefit of routine postoperative measurements of CA19-9, according with the American Society of Clinical Oncology's recommendations for the use of gastrointestinal tumor markers [34]. Similarly, postoperative monitoring recommended by European Society for Medical Oncology [4] and National Comprehensive Cancer Network [35] does not include CA19-9. In addition, the association between preoperative CA125 and CRC outcomes were marginally significant, which may be due to the small number of patients in the CA125-high group. A rising trend of CA125 after surgery indicated a poor prognosis, which may be related to liver metastasis [9] and peritoneal dissemination [8] of CRC. Whether to regard CA125 as a prognostic marker for postoperative follow-up of CRC needs to be verified by standardized randomized clinical trials. Although patients in other elevated groups accounted for lower proportions than the CEA elevated group in joint analyses, they were worthy of attention due to their specificity and high risks.
In the subgroup analysis, we observed that the HR for death in stage III patients with two rising markers was 3.9, lower than that in stage I or II patients with three rising markers, estimated to be 25 and 45, respectively. Likewise, results of a preoperative marker study demonstrated that stage III patients with fewer positive markers had longer disease-free survival and OS compared with stage II patients with more markers [30]. This suggested an interaction between the number of elevated tumor markers and pathological stage. The mechanisms of these interactions found in the subgroup analyses are unclear and need to be explored in further research. In application, the combined detection of multiple tumor markers may be helpful for clinicians to find out high-risk patients failed to be identified by clinicopathological characteristics alone, and then give focused attention and early treatment.
Our study had several strengths and limitations. The large-scale multicenter longitudinal cohort ensured our findings were robust in different conditions. The application of LCGMM characterized the longitudinal trajectories of tumor markers over the 36-month surveillance after surgery, in which at least four (median = 8) available marker measurements for each patient were used to reflect the overall profile. Compared with preoperative levels, trajectories can further reflect patient's response to surgery and adjuvant therapy, and provide dynamic information. And evaluating CEA, CA19-9 and CA125 jointly in terms of preoperative levels and trajectories provided more comprehensive information for the monitoring of tumor markers. The relative contribution of the trajectory joint group to predict OS and RFS exceeded the pathological stage, indicating the importance of dynamic measurements of multiple markers. And the poor prognosis of the CA125-rising and CA19-9-high group provided new ideas for further research on the clinical significance of CA19-9 and CA125 for the prognosis of colorectal cancer. One limitation of this study is that different immunoassays were performed in the measurements of tumor markers at the three centers. Though harmonization of the results obtained using the three immunoassays has not yet been achieved, system-specific reference intervals were considered. Moreover, the truncation of measurements in the trajectory analysis may change the highest point of the trajectory, but had little effect on the grouping of patients. A reported restrictive cubic spline showed that the HRs associated with prognosis of preoperative CEA remain unchanged when CEA exceeds 20ng/ml [17], supporting the rationality of truncation. Another limitation is the timing point, and the frequency of postoperative measurements were not controlled. We will further focus on the associations of these trajectory groups with CRC outcomes by prospective cohort. Besides, we did not control for other factors that can lead to false-positive elevation of these tumor markers, such as tobacco use [36], as this is hard to truthfully ascertain from patients [37]. In addition, diabetes, diseases of liver, pancreas and ovaries may also increase CEA, CA19-9 and CA125. If the interference of these factors cannot be ruled out in the practical application, false positives may be caused [38]. Patients lacking the Lewis antigen may show a false negative of CA19-9, without fucosyltransferase enzyme needed to produce CA 19-9, although the number is small (approximately 5% of the population) [39]. Clinicians should be cautious when using these markers to manage CRC patients. Though our study was multi-center, only data from South China was contained, which is not yet representative of the wider population due to heterogeneity between regions. A large-scale prospective cohort study need to be conducted for generalisability.
In conclusion, the longitudinal trajectories can provide additional prognostic information independent of preoperative levels, and the simultaneously dynamic measurements of CEA, CA19-9, and CA125 are recommended to monitor the prognosis of CRC patients.
5. Contributors
C.L., D.Y., Z.L., T.Z. did the concept and study design. C.L., Z.L., D.Z., X.P., H.P. and M.L. participated in the collection and assembly of data. C.L., D.Y., Z.L. and T.Z. designed the latent class growth mixed model. C.L., D.Y., Z.L., T.Z., B.F., and J.L. did the statistical analysis and gave interpreted the results. C.L. drafted the manuscript, and T.Z., Z.L. revised the manuscript. All authors reviewed and commented on the manuscript, and approved its final submission.
Data sharing statements
The data underlying this article cannot be shared publicly due to individuals’ privacy that participated in the study. The data will be shared on a reasonable request to the corresponding author.
Declaration of Competing Interest
All authors declare no competing interests.
Acknowledgments
This study is a joint effort of many investigators and staff members, and their contribution is gratefully acknowledged. We especially thank all patients who participated in this study. This research was funded by National Natural Science Foundation of China [grant numbers 81973147, 82001986, 81960592, 82073569, 81660545], the Outstanding Youth Science Fundation of Yunnan Basic Research Project [202001AW070021, 202101AW070001], and the Reserve Talent Project for Young and Middle-aged Acadamic and Technical Leaders [2012005AC160023], the Key Science Fundation of Yunnan Basic Research (202101AS070040), Innovative Research Team of Yunnan Province [202005AE160002], Yunnan digitalization, Development and Application of Biotic Resource [202002AA100007].
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2021.103706.
Contributor Information
Dingyun You, Email: youdingyun@qq.com.
Zhenhui Li, Email: lizhenhui621@qq.com.
Tao Zhang, Email: taozhang@sdu.edu.cn.
Appendix. Supplementary materials
References
- 1.Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017. JAMA Oncology. 2019;5(12):1749–1769. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National-Comprehensive-Cancer-Network(NCCN) National Comprehensive Cancer Network; Fort Washington, PA: 2020. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Colon Cancer. (Version 2.2020) [Google Scholar]
- 3.National-Comprehensive-Cancer-Network(NCCN) National Comprehensive Cancer Network; Fort Washington, PA: 2020. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Rectal Cancer. (Version 3.2020) [Google Scholar]
- 4.Argiles G, Tabernero J, Labianca R, et al. Localised Colon Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up. Ann Oncol. 2020;31(10):1291–1305. doi: 10.1016/j.annonc.2020.06.022. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Yang F, Peng J, Wang F, Lin Y, Jiang W, et al. High pretreatment serum CA19-9 level predicts a poor prognosis for patients with stage III colon cancer after curative resection and adjuvant chemotherapy. J Cancer. 2019;10(16):3810. doi: 10.7150/jca.31375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park IJ, Choi GS, Jun SH. Prognostic value of serum tumor antigen CA19-9 after curative resection of colorectal cancer. Anticancer Res. 2009;29(10):4303. [PubMed] [Google Scholar]
- 7.Webb A, Scott-Mackie P, Cunningham D, Norman A, Andreyev J, O'Brien M, et al. The prognostic value of CEA, βHCG, AFP, CA125, CA19-9 and C-erb B-2, (βHCG immunohistochemistry in advanced colorectal cancer. Annals of Oncology. 1995;6(6):581. doi: 10.1093/oxfordjournals.annonc.a059248. [DOI] [PubMed] [Google Scholar]
- 8.Huang CJ, Jiang JK, Chang SC, Lin JK, Yang SH. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine (Baltimore) 2016;95(47):e5177. doi: 10.1097/MD.0000000000005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mavligit GM, Estrov Z. CA 125: a clinically useful tumor marker in the management of colorectal carcinoma metastatic to the liver in patients with normal carcinoembryonic antigen. Am J Clin Oncol. 2000;23(2):213. doi: 10.1097/00000421-200004000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Stiksma J, Grootendorst DC, van der Linden PW. CA 19-9 as a marker in addition to CEA to monitor colorectal cancer. Clin Colorectal Cancer. 2014;13(4):239. doi: 10.1016/j.clcc.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Duffy MJ, van Dalen A, Haglund C, Hansson L, Holinski-Feder E, Klapdor R, et al. Tumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical use. Eur J Cancer. 2007;43(9):1348. doi: 10.1016/j.ejca.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 12.Yang XQ, Li Y, Chen C, Peng CW, Liu SP, Liu Y. Preoperative serum carbohydrate antigen 125 level is an independent negative prognostic marker for overall survival in colorectal cancer. Med Oncol. 2011;28(3):789. doi: 10.1007/s12032-010-9518-z. [DOI] [PubMed] [Google Scholar]
- 13.Yang XQ, Chen C, Wang FB, Peng CW, Li Y. Preoperative serum carcinoembryonic antigen, carbohydrate antigen19-9 and carbohydrate antigen 125 as prognostic factors for recurrence-free survival in colorectal cancer. Asian Pac J Cancer Prev. 2011;12(5):1251. [PubMed] [Google Scholar]
- 14.Baqar AR, Wilkins S, Staples M, Angus Lee CH, Oliva K, McMurrick P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int J Surg. 2019;64:10–15. doi: 10.1016/j.ijsu.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Nakamura Y, Shida D, Tanabe T, Takamizawa Y, Imaizumi J, Ahiko Y, et al. Prognostic impact of preoperatively elevated and postoperatively normalized carcinoembryonic antigen levels following curative resection of stage I-III rectal cancer. Cancer Med. 2020;9(2):653–662. doi: 10.1002/cam4.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liska V, Holubec L, Jr., Treska V, Skalicky T, Sutnar A, Kormunda S, et al. Dynamics of serum levels of tumour markers and prognosis of recurrence and survival after liver surgery for colorectal liver metastases. Anticancer Res. 2007;27(4C):2861. [PubMed] [Google Scholar]
- 17.Li Z, Li C, Pu H, Pang X, Wang Y, Zhang D, et al. Trajectories of perioperative serum carcinoembryonic antigen and colorectal cancer outcome: A retrospective, multicenter longitudinal cohort study. Clin Transl Med. 2021;11(2):e293. doi: 10.1002/ctm2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z, Li S, Liang Y, Pu H, Tu C, Wu Z, et al. Predictive Value of Postoperative Peripheral CD4+ T Cells Percentage in Stage I-III Colorectal Cancer: A Retrospective Multicenter Cohort Study of 1028 Subjects. Cancer Manag Res. 2020;12:5505. doi: 10.2147/CMAR.S259464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WS/T 645. 2- Reference intervals for common clinical immunology tests— Part 2: Serum α-fetoprotein, carcinoembryonic antigen, carbohydrate antigen 19-9. carbohydrate antigen 15-3, carbohydrate antigen. 2018;125 [Google Scholar]
- 20.Proust-Lima C, Philipps V, Liquet B. Estimation of extended mixed models using latent classes and latent processes: the R package lcmm. J Stat Softw. 2015;78(2):1–56. [Google Scholar]
- 21.Heather A, Natasha C, Amanda T, Patrick G. Latent Class Growth Modelling: A Tutorial. Tutorials in Quantitative Methods for Psychology. 2009;5(1):11–24. [Google Scholar]
- 22.Buscot MJ, Thomson RJ, Juonala M, Sabin MA, Burgner DP, Lehtimaki T, et al. Distinct child-to-adult body mass index trajectories are associated with different levels of adult cardiometabolic risk. Eur Heart J. 2018;39(24):2263. doi: 10.1093/eurheartj/ehy161. [DOI] [PubMed] [Google Scholar]
- 23.Pu H XP, Chen Y, Zhao Y, Ye X, Lu G, Zhang D, Li Z. Relationship Between Preoperative and Postoperative Serum Carcinoembryonic Antigen and Prognosis of Patients with Stage I–III Rectal Cancer: A Retrospective Study of a Multicentre Cohort of 1022 Rectal Cancer Patients. Cancer Management and Research. 2021;13:2643. doi: 10.2147/CMAR.S290416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HG YS, Han YD, Cho MS, Min BS, Lee KY, Kim NK, Hur H. Association of perioperative serum carcinoembryonic antigen level and recurrence in low-risk stage IIA colon cancer. PLoS ONE. 2021;16(6) doi: 10.1371/journal.pone.0252566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song S, Hong JC, McDonnell SE, Koong AC, Minsky BD, Chang DT, et al. Combined modality therapy for rectal cancer: the relative value of posttreatment versus pretreatment CEA as a prognostic marker for disease recurrence. Ann Surg Oncol. 2012;19(8):2471. doi: 10.1245/s10434-012-2266-x. [DOI] [PubMed] [Google Scholar]
- 26.Slentz K, Senagore A, Hibbert J, Mazier WP, Talbott TM. Can preoperative and postoperative CEA predict survival after colon cancer resection? Am Surg. 1994;60(7):528. discussion 31-2. [PubMed] [Google Scholar]
- 27.Lin JK, Lin CC, Yang SH, Wang HS, Jiang JK, Lan YT, et al. Early postoperative CEA level is a better prognostic indicator than is preoperative CEA level in predicting prognosis of patients with curable colorectal cancer. Int J Colorectal Dis. 2011;26(9):1135. doi: 10.1007/s00384-011-1209-5. [DOI] [PubMed] [Google Scholar]
- 28.Wood CB, Ratcliffe JG, Burt RW, Malcolm AJ, Blumgart LH. The clinical significance of the pattern of elevated serum carcinoembryonic antigen (CEA) levels in recurrent colorectal cancer. Br J Surg. 1980;67(1):46. doi: 10.1002/bjs.1800670114. [DOI] [PubMed] [Google Scholar]
- 29.Hung H YJ, Chiang J, Hsieh P, Chiang S, Lai C, Tasi W, Yeh C. Why recurrence was initially suspected during colorectal cancer postoperative surveillance?: A retrospective analysis. Medicine (Baltimore) 2020;99(43):e22803. doi: 10.1097/MD.0000000000022803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.You W, Sheng N, Yan L, Chen H, Gong J, He Z, et al. The difference in prognosis of stage II and III colorectal cancer based on preoperative serum tumor markers. J Cancer. 2019;10(16):3757. doi: 10.7150/jca.31660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibutani M, Maeda K, Nagahara H, Ohtani H, Sakurai K, Toyokawa T, et al. Significance of CEA and CA19-9 combination as a prognostic indicator and for recurrence monitoring in patients with stage II colorectal cancer. Anticancer Res. 2014;34(7):3753. [PubMed] [Google Scholar]
- 32.Lipska L, Visokai V, Levy M, Svobodova S, Kormunda S, Finek J. Tumor markers in patients with relapse of colorectal carcinoma. Anticancer Res. 2007;27(4A):1901. [PubMed] [Google Scholar]
- 33.Lakemeyer L SS, Wittau M, Henne-Bruns D, Kornmann M, Lemke J. Diagnostic and Prognostic Value of CEA and CA19-9 in Colorectal Cancer. Diseases. Diseases. 2021;9(1):21. doi: 10.3390/diseases9010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Locker GY, Hamilton S, Harris J, Jessup JM, Kemeny N, Macdonald JS, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol. 2006;24(33):5313. doi: 10.1200/JCO.2006.08.2644. [DOI] [PubMed] [Google Scholar]
- 35.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang CS CC, Huang LK, Wang WS, Yang SH. Prognostic value of postoperative serum carcinoembryonic antigen levels in colorectal cancer patients who smoke. PLoS One. 2020;15(6) doi: 10.1371/journal.pone.0233687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Konishi T SY, Hsu M, Tufts L, Jimenez-Rodriguez R, Cercek A, Yaeger R, Saltz L, Smith JJ, Nash GM, Guillem JG, Paty PB, Garcia-Aguilar J, Gonen M, Weiser MR. Association of Preoperative and Postoperative Serum Carcinoembryonic Antigen and Colon Cancer Outcome. JAMA Oncol. 2018;4(3):309–315. doi: 10.1001/jamaoncol.2017.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang CS CC, Huang LK, Wang WS, Yang SH. Postoperative serum carcinoembryonic antigen levels cannot predict survival in colorectal cancer patients with type II diabetes. J Chin Med Assoc. 2020;83(10):911. doi: 10.1097/JCMA.0000000000000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scarà S BP, Scatena R. CA 19-9: Biochemical and Clinical Aspects. Adv Exp Med Biol. 2015;867:247. doi: 10.1007/978-94-017-7215-0_15. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.