Figure 1.
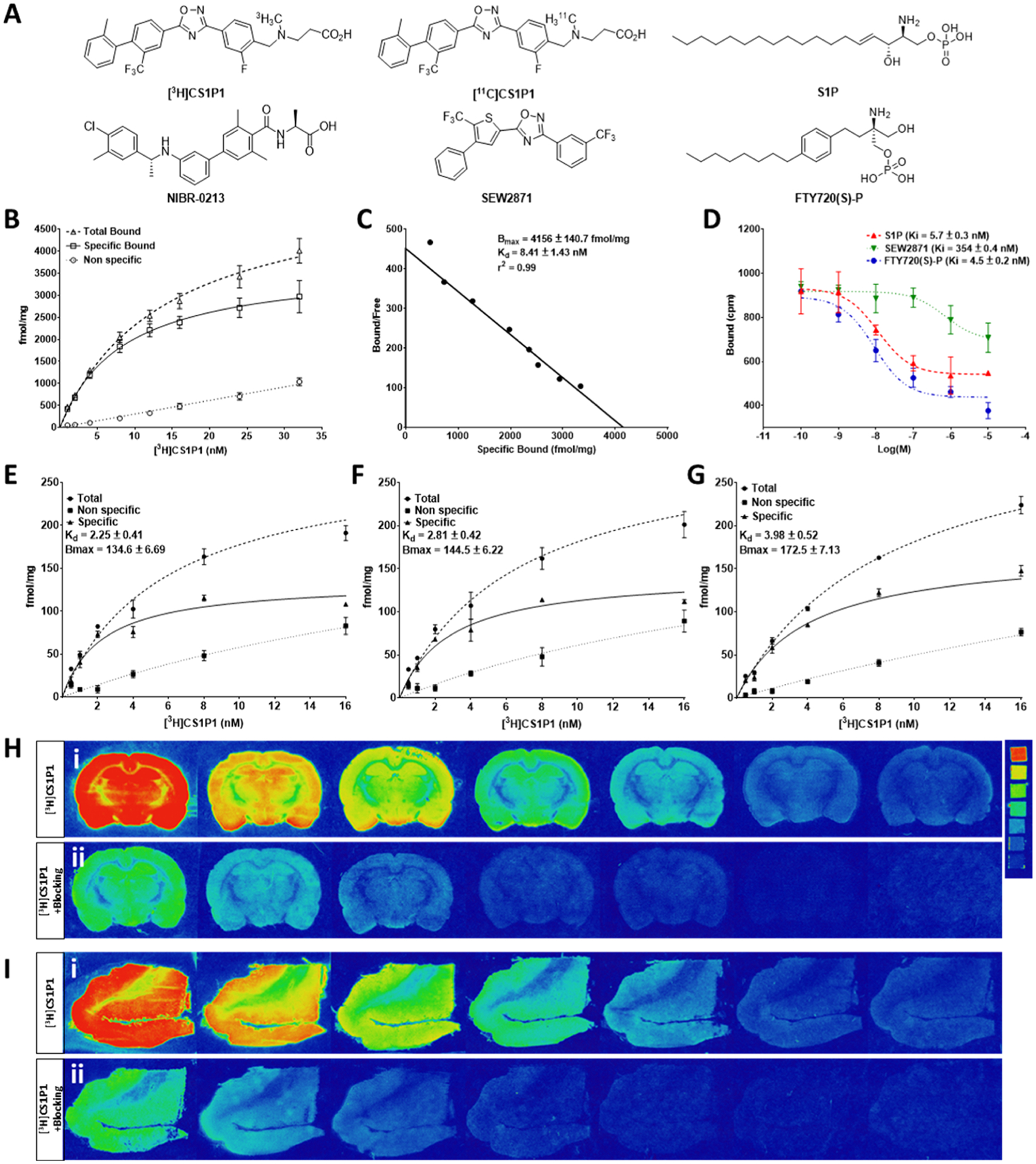
In vitro characterization of potency and specificity of [3H]CS1P1 toward S1PR1. (A) Chemical structures of CS1P1 and other compounds used in this study. (B–D) Saturation and competition binding assay of [3H]CS1P1 with recombinant human S1PR1 membrane: saturation binding assay showed a high affinity of [3H]CS1P1 to hS1PR1 with a Kd of 8.41 nM (B–C); [3H]CS1P1 can compete with the known potent S1PR1 ligand and S1P with a Ki value of 5.7 nM for S1P, 4.5 nM for FTY720(S)-P, and 354 nM for SWE2871 (D). (E–I) Saturation binding autoradiograph analysis of [3H]CS1P1 in the human and rat brain: (E) Saturation binding assay showed a high affinity of [3H]CS1P1 to the gray matter of the human frontal cortex with a Kd of 8.54 nM; (F) saturation binding assay showed a high affinity of [3H]CS1P1 to the cortex region of the rat brain with a Kd of 5.94 nM; (G) saturation binding assay showed a high affinity of [3H]CS1P1 to the hippocampus region of the rat brain with a Kd of 6.57 nM; (H) representative images of saturation binding autoradiograph analysis of [3H]CS1P1 in the rat brain and image of tritium ART-123 standard (activities of standard from top to bottom in μCi/g: 489.1, 243, 138.1, 63.1, 36.3, 16.6, and 8); (I) representative images of saturation binding autoradiograph analysis of [3H]CS1P1 in the human frontal cortex.
