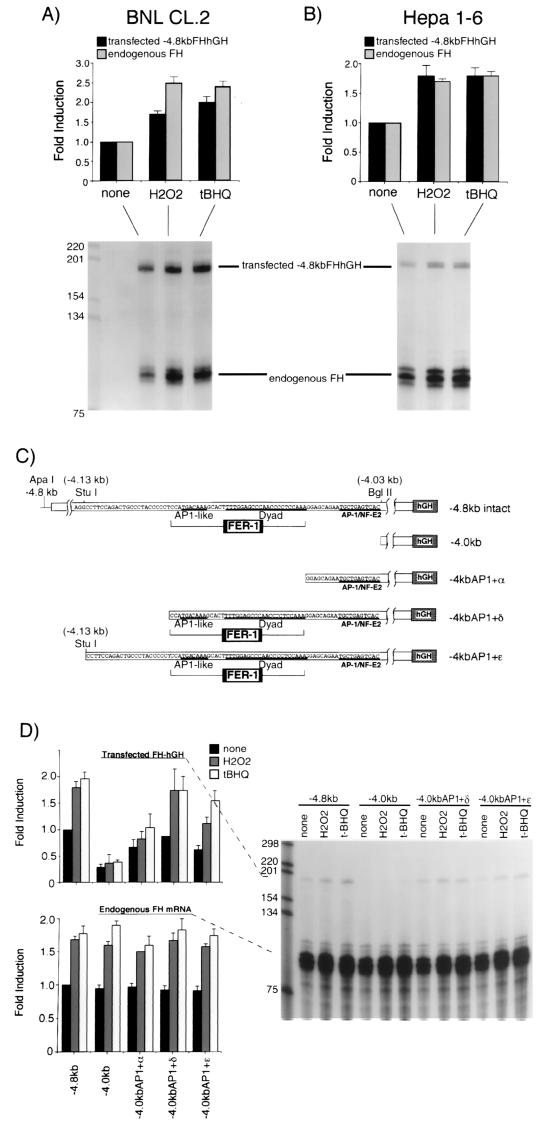
FIG. 2.
The cis-acting element responsible for transcriptional activation of the mouse ferritin H gene by H2O2 or t-BHQ is located 4.1 kb upstream to the transcriptional initiation site. BNL CL.2 (A) or Hepa1-6 cells (B) were transfected with 15 μg of −4.8kbFH-hGH. After 36 to 40 h, the cells were treated with H2O2 (750 μM for BNL CL.2 cells and 250 μM for Hepa1-6 cells) or t-BHQ (250 μM for BNL CL.2 cells and 100 μM for Hepa1-6 cells) for 8 to 10 h. A 10-μg portion of total RNA isolated from each treatment was subjected to RNase protection assays as described in Materials and Methods to analyze the expression of transfected −4.8kbFHhGH (the protected RNA band is 190 bases) and endogenous ferritin H mRNA (the protected RNA band is 86 bases) simultaneously. Each transfected and endogenous ferritin H band without treatment was defined as 1.0, and the results from seven (BNL CL.2) and eight (Hepa1-6) independent experiments and standard errors are shown. (C) Schematic representation of ferritin H-hGH constructs used in this experiment. A basal enhancer element of the mouse ferritin H gene, FER-1 (42), and a proximal AP1/NF-E2 site are indicated in the diagram. (D) A 15-μg portion of each hGH construct was transiently transfected into Hepa1-6 cells. After 36 to 40 h, the cells were treated with 250 μM H2O2 or 100 μM t-BHQ for 8 to 10 h. A 10-μg portion of total RNA isolated from each sample was subjected to RNase protection assays to analyze the expression of transfected ferritin H-hGH and endogenous ferritin H mRNA simultaneously. Each transfected and endogenous ferritin H band in the cells transfected with −4.8kbFHhGH without treatment was defined as 1.0, and the results from six independent experiments and standard errors are shown.