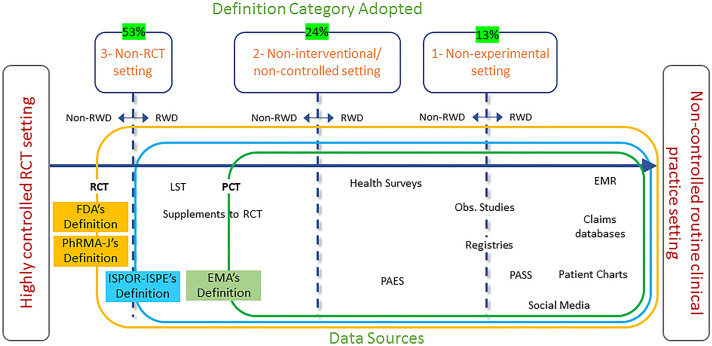
Fig. 2.
Real-world data definitions and data sources.
Adapted from Makady et al. [16] with permission from Value Health 2017; 20(7):858-65. Copyright© 2017 International Society for Pharmacoeconomics and Outcomes Research (ISPOR). Published by Elsevier Inc. All rights reserved. EMR electronic medical record, LST large simple trial, Obs. Observational, PAES post-authorization efficacy study, PASS post-authorization safety studies, PCT pragmatic clinical trial, RCT randomized clinical trial, RWD real-world data, EMA Europe Medical Agency, FDA US Food and Drug Administration, ISPOR the International Society of Pharmacoeconomics and Outcomes Research, ISPE the International Society of Pharmacoepidemiology