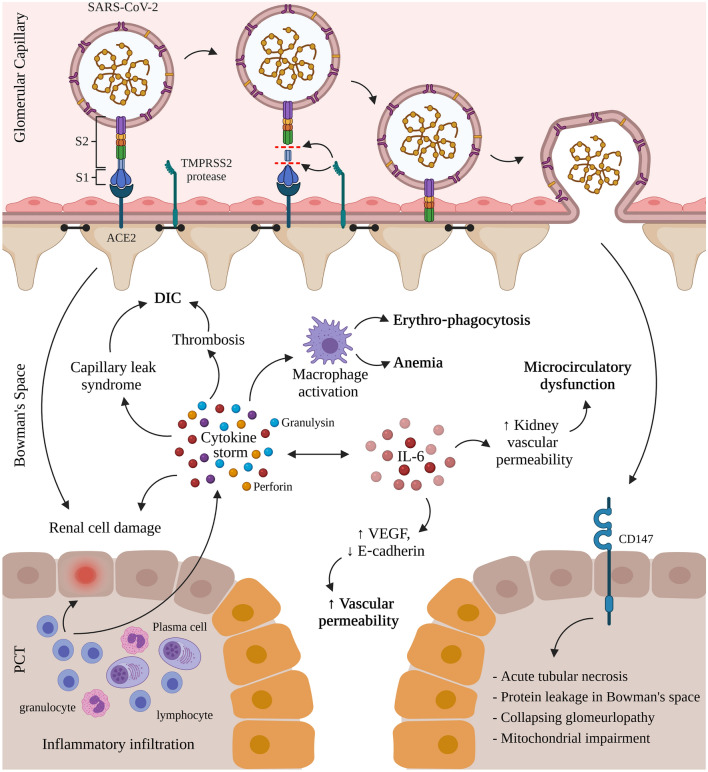
Fig. 1.
Mechanisms of renal entry and kidney injury in COVID-19 infection. COVID-19 infection in humans proceeds by the interaction of the receptor-binding domain (RBD) of the viral spike protein with the cell-surface angiotensin-converting enzyme II (ACE2). This is followed by the proteolytic cleavage of the spike protein through proteases like the transmembrane protease serine 2 (TMPRSS2). The virus interacts with CD147, expressed on the proximal convoluted tubules (PCT) of the nephron and on infiltrating inflammatory cells, resulting in acute tubular necrosis, protein leakage in Bowman’s capsule, collapsing glomerulopathy, and mitochondrial impairment. Simultaneously, the activated lymphocytes from the inflammatory infiltrates (lymphocytes, plasma cells and eosinophils) in the renal interstitium destroy renal cells and induce a cytokine storm of perforin, granulysin, and proinflammatory cytokines. The cytokine storm activates macrophages leading to erythro‐phagocytosis and anemia, induces capillary leak syndrome and thrombosis both linked to disseminated intravascular coagulation (DIC), and contributes to renal cell damage also caused by direct renal infection. Oversecretion of key cytokine, interleukin-6 (IL‐6), that binds the IL‐6 receptor and activates the vascular endothelial growth factor (VEGF), decreases the expression of E‐cadherin, increases vascular permeability, shock, and MOD while increasing kidney vascular permeability and microcirculatory dysfunction. (Created with Biorender.com)