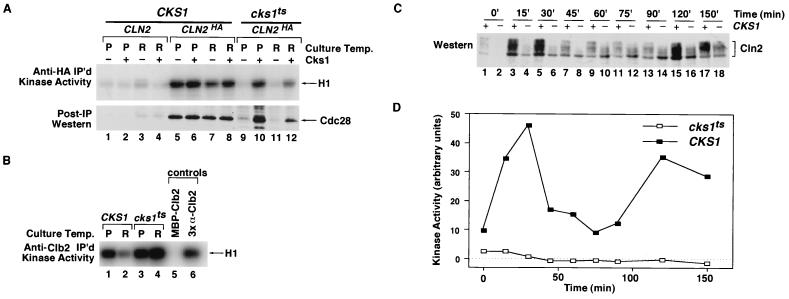
FIG. 5.
Mutant cks1-38 cells fail to assemble active Cln2-Cdc28 protein kinase complexes. (A) Absence of active Cln23×HA-Cdc28 complexes in cks1-38 cell extract. Total cell extract proteins (3 mg) prepared from CLN2 CKS1, CLN23×HA CKS1, and CLN23×HA cks1-38 cells grown at either the permissive (P; 24°C) or restrictive (R; 38.5°C) temperature were immunoprecipitated (IP) with anti-HA monoclonal antibody 12CA5, and immunoprecipitates were divided in half and assayed for their content of histone H1 kinase activity (top) or Cdc28 antigen (bottom). Samples in even-numbered lanes were supplemented with 3.9 μg of purified Cks1 prior to the immunoprecipitation step. Relative kinase activities in lanes 1 to 12 (top) are 1, 1, 2, 1, 25, 29, 15, 23, 2, 20, 2, and 8, respectively. (B) cks1-38 extracts contain high levels of Clb2-Cdc28 kinase activity. Total cell extract proteins (1 mg) from the experiment described for panel A were incubated with anti-Clb2 affinity-purified polyclonal antibodies, and immunoprecipitates were assayed for their content of histone H1 kinase activity. MBP-Clb2, same as sample shown in lane 1, except 20 μg of purified MBP-Clb2 was added prior to the immunoprecipitation step; 3× α-Clb2, same as sample shown in lane 1, except threefold more antibody was used. These two controls indicate that immunoprecipitation was specific and that the antibody was used at saturating levels for the assays depicted in lanes 1 to 4. The relative protein kinase activities in lanes 1 to 6 are 18, 6, 24, 36, 1, and 13, respectively. (C) Accumulation of phosphorylated Cln23×HA is strongly delayed in cks1-38 cells released from a pheromone arrest. CKS1 (odd-numbered lanes) and cks1-38 (even-numbered lanes) cultures were arrested in G1 phase with α-factor, shifted to 38.5°C for 1 h, and then released by being washed into medium lacking α-factor at 38.5°C. Lysates (100 μg) prepared from cells withdrawn at the indicated times were fractionated by SDS-PAGE and immunoblotted with anti-HA monoclonal antibody 12CA5 to detect Cln23×HA. Note that both the level and phosphorylation state of Cln2 are diminished in cks1-38 cells. (D) Cln23×HA-Cdc28 activity fails to accumulate in cks1-38 cells released from a pheromone arrest. Samples (3 mg) from the experiment shown in panel C were immunoprecipitated with 12CA5. Immunoprecipitates were assayed for their content of histone H1 kinase activity, which was evaluated by SDS-PAGE followed by phosphorimaging.