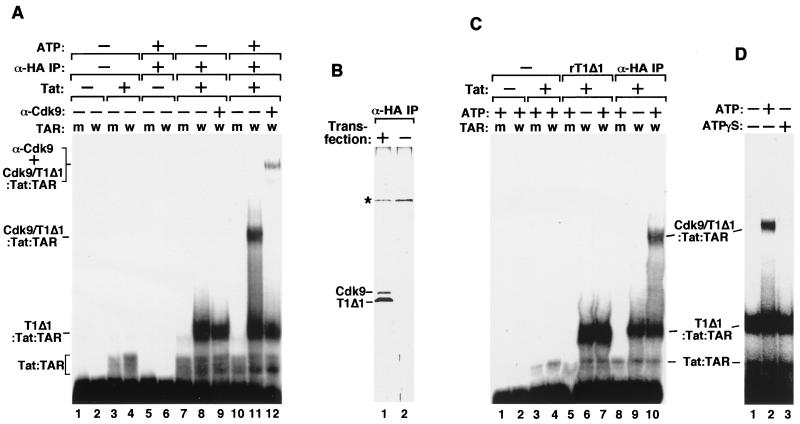
FIG. 1.
Requirement of ATP hydrolysis for formation of a stable P-TEFb–Tat–TAR ribonucleoprotein complex. (A) The Cdk9–HA-CycT1Δ1 heterodimer was affinity purified from 293T cells transiently transfected with an HA-tagged CycT1Δ1 cDNA by α-HA IP followed by HA peptide elution. The purified fraction containing the heterodimer and free HA-CycT1Δ1 was incubated with 32P-labeled wild-type TAR RNA (lanes labeled w) or the loop mutant TAR+31/+34 (lanes labeled m) in the absence (−) or presence (+) of Tat and/or ATP as indicated. The reactions were analyzed by EMSA. Lanes 9 and 12 also contained anti-Cdk9 antibodies (α-Cdk9), which caused the retardation of the Cdk9–HA-CycT1Δ1–Tat–TAR complex and also partially destabilized the complex, probably because of the polyclonal nature of the antibodies. (B) Silver-stained SDS-polyacrylamide gel showing the presence of Cdk9–HA-CycT1Δ1 and free HA-CycT1Δ1 in the α-HA IP fraction (lane 1). A nonspecific protein (∗) was revealed in a control fraction prepared in parallel from untransfected 293T cells (lane 2). (C) Free HA-CycT1Δ1 present in the α-HA IP fraction formed an ATP-independent complex with Tat and TAR in gel shift reactions. Recombinant CycT1Δ1 (rT1Δ1) was used as a reference (lanes 5 to 7) for determining the identity of the ATP-independent complex formed with the α-HA IP fraction (lanes 8 to 10). (D) Requirement of ATP hydrolysis for P-TEFb–Tat–TAR assembly. All reaction mixtures contained the α-HA IP fraction, Tat, and 32P-labeled wild-type TAR RNA. [γ-S]ATP was used in place of ATP in lane 3.