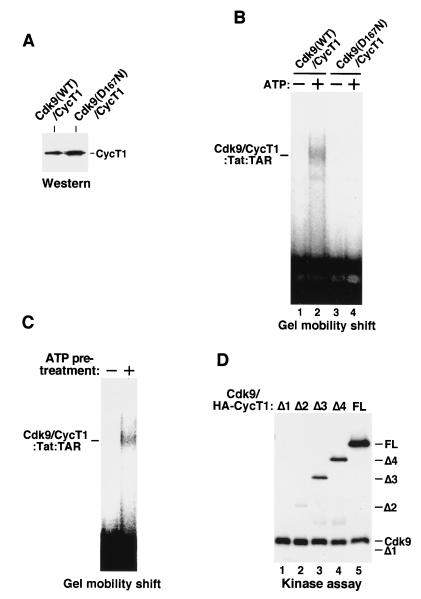
FIG. 3.
Autophosphorylation of Cdk9 is required for efficient P-TEFb–Tat–TAR complex formation. (A) P-TEFb complexes containing either HA-tagged wild-type Cdk9 (WT) or a kinase-defective Cdk9 (D167N) were affinity purified by α-HA IP and normalized for their CycT1 levels by Western blotting with anti-CycT1 antibodies. (B) The kinase activity of Cdk9 is required for P-TEFb–Tat–TAR formation. The two P-TEFb complexes prepared for panel A were compared in a gel mobility shift assay for forming P-TEFb–Tat–TAR complexes in the presence (+) or absence (−) of ATP. Since the HA tag was attached to Cdk9, no free CycT1 was present in these α-HA IP fractions. (C) Autophosphorylation of P-TEFb is required for P-TEFb–Tat–TAR complex formation. Purified Cdk9-HA–CycT1 immobilized on anti-HA antibody beads was incubated with ATP in a kinase reaction mixture. Upon removal of ATP, the phosphorylated complex was eluted from the beads and incubated with Tat and 32P-labeled wild-type TAR RNA in a reaction mixture without ATP. An equal amount of unphosphorylated P-TEFb was used as a control. (D) Phosphorylation of CycT1 by Cdk9 is not required for P-TEFb–Tat–TAR formation. Cdk9–HA-CycT1FL and Cdk9–HA-CycT1Δ1 to -Δ4 complexes were prepared, and their levels were normalized as described for Fig. 2B. Equal amounts of these complexes were analyzed in in vitro kinase reactions. Note that HA-CycTΔ1 was not phosphorylated by Cdk9. However, Cdk9–HA-CycT1Δ1 formed an ATP-dependent complex with Tat-TAR.