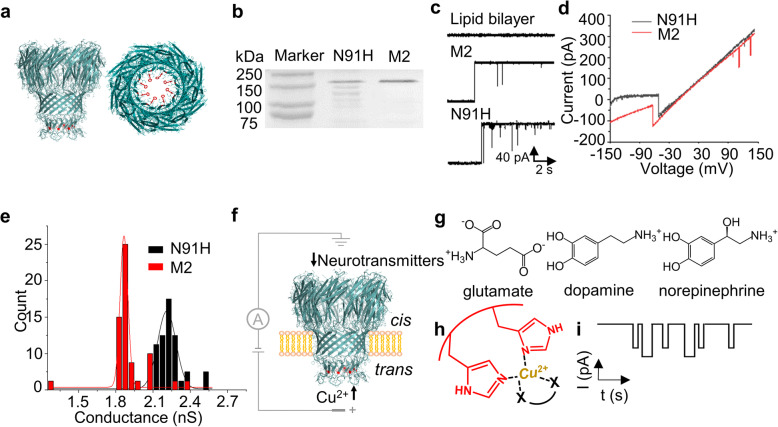
Fig. 1.
Electrophysiological properties of the engineered M2MspA-N91H and scheme of neurotransmitters detection. a Side-view (left) and top-view (right) of M2MspA-N91H structure modeled by SWISS-MODEL. b SDS-PAGE analysis of M2MspA-N91H and M2MspA. N91H represents M2MspA-N91H, and M2 represents M2MspA. c Typical current traces of blank, M2MspA and M2MspA-N91H nanopore inserting into lipid bilayer in 1 M KCl, pH 7.5, 50 mV. d I-V curves of M2MspA-N91H and M2MspA at scanning potential from − 150 to 150 mV. e Conductance histograms of M2MspA-N91H and M2MspA nanopore inserting into lipid bilayer in 1 M KCl, pH 7.5 (n = 35). f Experimental setup. The mutant protein M2MspA-N91H is incorporated into lipid bilayer from the cis (grounded) side of the setup. Cu2+ and neurotransmitters are added to trans and cis side, respectively, which both move towards the constriction region of M2MspA-N91H under a positive potential. g Ionization structures of three neurotransmitters: glutamate, dopamine and norepinephrine. h The principle of detection of neurotransmitters. Cu2+ non-covalently binds to the two nearby histidine residues at the constriction region of M2MspA-N91H firstly, then neurotransmitters which can interact with Cu2+ are detected. i Idealized current signals caused by different kinds of neurotransmitters binding reversibly to the Cu2+-M2MspA-N91H complex