ABSTRACT
Background
This meta-analysis of randomized controlled trials (RCTs) investigated the usefulness of Janus kinase (JAK) inhibitors among hospitalized patients with COVID-19.
Methods
PubMed, Web of Science, the Cochrane Library, and Ovid MEDLINE were searched for RCTs published before 7 September 2021. Only RCTs that compared the clinical efficacy and safety of JAK inhibitors with other alternative treatments or placebos in the treatment of hospitalized patients with COVID-19 were included.
Results
Overall, patients receiving JAK inhibitors exhibited a lower 28-day mortality rate than the control group (risk ratio [RR], 0.60; 95% CI, 0.47–0.77; I2 = 0%). Compared with the control group, the study group also had a lower 14-day mortality rate (RR, 0.60; 95% CI, 0.42–0.85; I2 = 0%), a higher rate of clinical improvement (RR, 1.05; 95% CI, 1.02–1.09; I2 = 0%), and less need of mechanical ventilation or extracorporeal membrane oxygenation (RR, 0.64; 95% CI, 0.50–0.84; I2 = 0%). Finally, JAK inhibitor use was associated with a similar risk of adverse events and infections as that observed in the control group.
Conclusions
JAK inhibitors can help reduce mortality and improve clinical outcomes among hospitalized patients with COVID-19. Additionally, JAK inhibitors can be used safely in this clinical entity.
KEYWORDS: COVID-19, Janus kinase inhibitor, mortality, SARS-CoV-2
1. Introduction
With more than 220 million confirmed cases, COVID-19 is a global health emergency [1]. Although patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can present as asymptomatic or with mild infection, some can progress to severe or critical COVID-19 [2], which has caused more than 4 million deaths as of early September 2021 [1]. Currently, only systemic corticosteroid and tocilizumab have been demonstrated to reduce mortality in patients with COVID-19 [3,4]. Therefore, effective treatment for hospitalized patients with COVID-19 remains limited [5,6].
Among hospitalized patients with COVID-19, a hyperinflammatory response may develop and can be associated with additional multiple organ dysfunctions [7,8]. Because Janus kinase (JAK) inhibitors, well-known anti-inflammatory agents, are indicated in many autoimmune diseases [9–12], they have been repurposed as a potential intervention for patients with COVID-19 [13]. Thus, the clinical efficacy and safety of JAK inhibitors for the treatment of patients with COVID-19 have been evaluated in several randomized controlled trials (RCTs) [14–18]. However, the benefit of JAK inhibitors was not consistently demonstrated among these RCTs [14–18].Although meta-analyses have investigated and provided evidence concerning the usefulness of JAK inhibitors for patients with COVID-19, most of these analyses were based on small numbers of RCTs with limited study populations [19–22]. Recently, a large RCT of 1525 participants studied outcomes of JAK inhibitor treatment among hospitalized adults with COVID-19 [17]. Therefore, we conducted this new meta-analysis using updated data to provide robust and timely evidence.
2. Methods
2.1. Study search and selection
We searched PubMed, Web of Science, the Cochrane Library, and Ovid MEDLINE for RCTs published before 7 September 2021. The following search terms were used: ‘Janus kinase,’ ‘JAK,’ ‘ruxolitinib,’ ‘tofacitinib,’ ‘oclacitinib,’ ‘baricitinib,’ ‘peficitinib,’ ‘fedratinib,’ ‘upadacitinib,’ ‘filgotinib,’ ‘delgocitinib,’ ‘nezulcitinib,’ ‘Covid-19,’ ‘SARS-CoV-2,’ ‘coronavirus,’ ‘2019-nCoV,’ and ”corona-virus.” Only RCTs that compared the clinical efficacy and safety of JAK inhibitors with other alternative treatments or placebos in the treatment of hospitalized patients with COVID-19 were included. Studies were included if they met the following criteria: (1) examined patients with COVID-19; (2) used JAK inhibitor as the intervention; (3) used other treatment options, or placebo as control group; (4) study designed as a RCT; and (5) the data regarding clinical efficacy and risk of adverse events (AEs) as study outcomes was available. We excluded case reports, case series, observational studies, and retrospective cohort studies. Two authors (SHL and LCL) independently reviewed the identified abstracts and selected articles for full review. Disagreements were resolved by the third author (WTL). For each included study, we extracted the following data: year of publication, study design, JAK inhibitor regimen, clinical outcomes, and risk of adverse events (AEs) from the included studies. This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [23], and the study protocol is registered in PROSPERO (CRD42021278061).
2.2. Outcome measurement
The primary outcome of this meta-analysis was 28-day all-cause mortality, and the secondary outcomes were 14-day mortality, the rate of clinical improvement, the need for mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO), and the risk of AEs and infections.
2.3. Data analysis
The Cochrane risk of bias tool [24] was used by 2 authors (SHL and SPC) to assess the quality of the included RCTs and their associated risk of bias. Any discrepancies between these 2 authors were solved by a third author (CCL). Statistical analyses were performed using Review Manager version 5.3 with the random-effects model. Pooled risk ratios (RRs) with 95% CIs were calculated.
3. Results
3.1. Study selection
The search results yielded a total of 638 studies from the online databases (PubMed: 232; Web of Science: 202; the Cochrane Library: 60; and Ovid MEDLINE: 144, Appendix A), of which 331 studies were excluded as duplicates. Moreover, 294 studies were deemed irrelevant after the title and abstract were screened, and 8 studies were deemed irrelevant after the full text was screened. Thus, 5 RCTs [14–18] were included in this meta-analysis (Figure 1).
Figure 1.
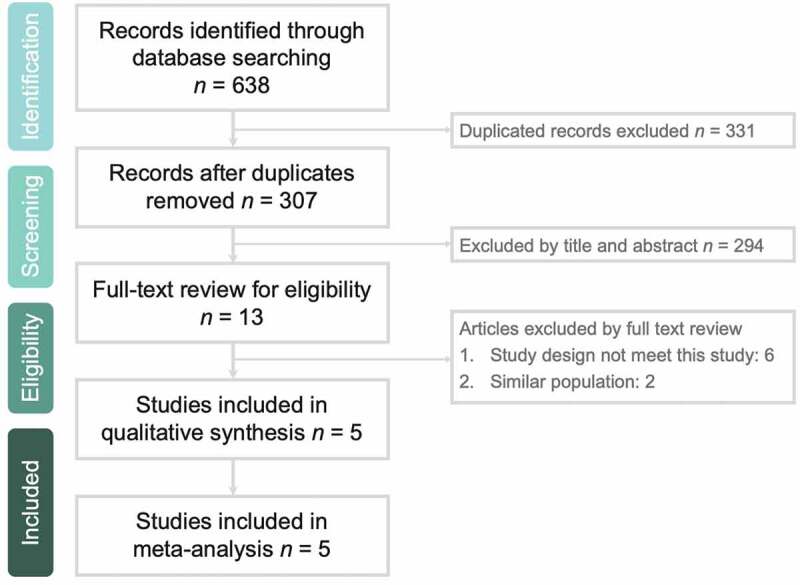
Flow diagram of study identification and assessment for eligibility
3.2. Study characteristics
Among the 5 RCTs, 2 were phase 2 studies [14,18] focusing on patients with severe COVID-19, and 3 were phase 3 studies focusing on hospitalized patients with COVID-19 [15–17] (Table 1). All RCTs were multicenter studies, and 3 of them were international studies [16–18]. Four RCTs used oral JAK inhibitors, namely baricitinib (n = 2) [16,17], tofacitinib (n = 1) [15], and ruxolitinib (n = 1) [14], as interventions. Only one RCT [17] examined an inhaled JAK inhibitor – nezulcitinib. The study group treated with JAK inhibitors and the control group consisted of 1462 and 1451 patients, respectively. Except for the unknown risk of detection biases in the study by Cao et al. [14], and high risk of selection and detection bias in the study by Singh et al. [18], the other studies exhibited a low risk of bias in all fields (Figure 2).
Table 1.
Characteristics of included studies
| Author, year | Study design | Study sites | Participants | Janus kinase inhibitor (route) | Comparator | Number of participants in the study group | Number of participants in the control group |
|---|---|---|---|---|---|---|---|
| Phase 2 study | |||||||
| Cao et al. [14] | Randomized single-blinds trial | 3 hospitals in China | Adult patients with severe COVID-19 | Ruxolitinib (oral) with 5 mg twice daily | Vitamin C | 20 | 21 |
| Singh et al. [18] | Randomized double-blind, placebo-controlled trial | Multicenter in UK, Moldova, and Ukraine | Adult patients with severe COVID-19 | Nezulcitinib (inhalation) with 1, 3 10 mg daily for up to 7 days | Placebo | 19 | 6 |
| Phase 3 study | |||||||
| Guimarães et al. [15] | Randomized, double-blind, placebo-controlled trial | 15 sites in Brazil | Hospitalized adults with COVID-19 | Tofacitinib (oral) with 10-mg twice daily for 14 days or until hospital discharge | Placebo | 144 | 145 |
| Kalil et al. [16] | Randomized double-blind, placebo-controlled trial | 67 centers in 8 countries | Hospitalized adults with COVID-19 | Baricitinib (oral) with 4-mg daily dose for 14 days or until hospital discharge | Placebo | 515 | 518 |
| Marconi et al. [17] | Randomized double-blind, placebo-controlled trial | 101 centers in 12 countries | Hospitalized adults with COVID-19 | Baricitinib (oral) with 4-mg daily dose for 14 days or until hospital discharge | Placebo | 764 | 761 |
Figure 2.
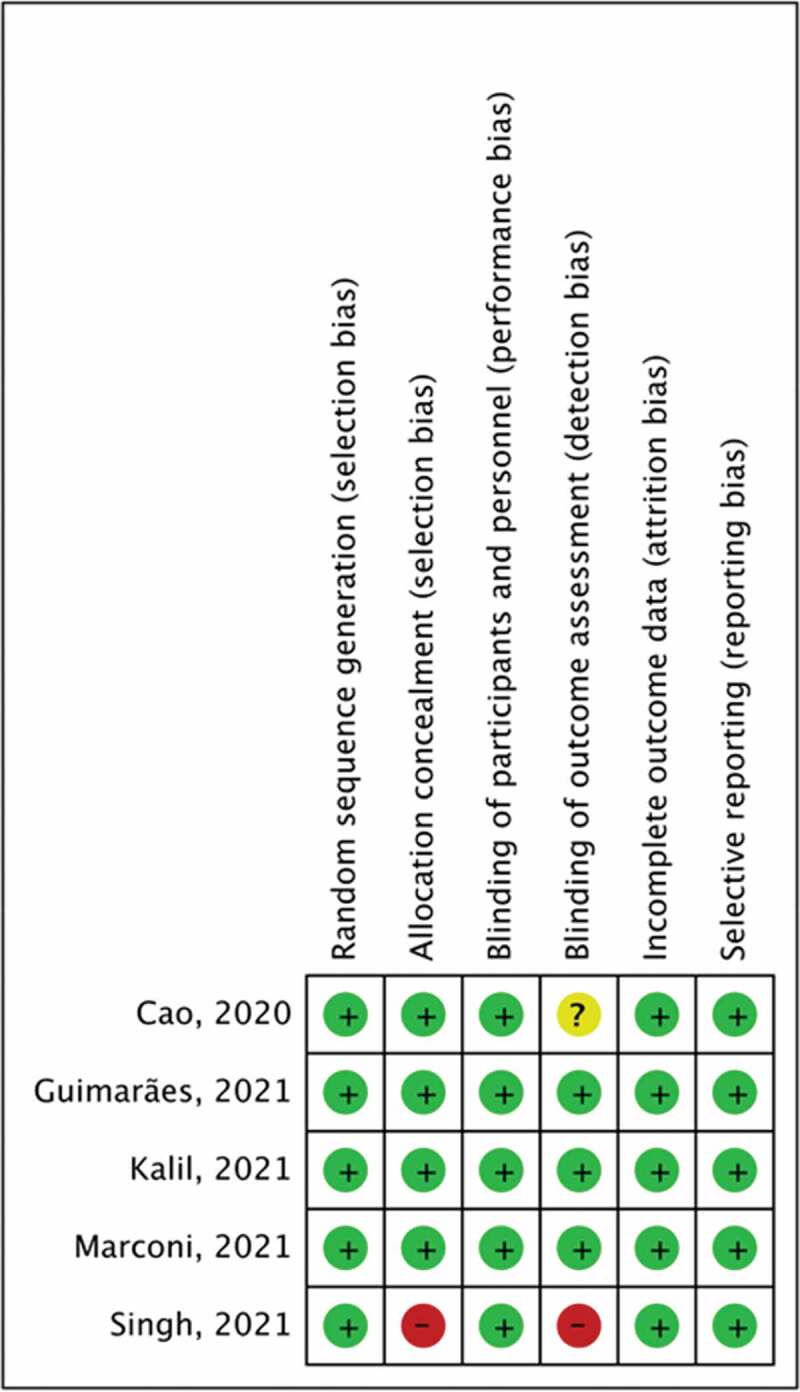
Summary of risk of bias assessment
3.3. Primary outcome
The 28-day mortality rate was 6.2% (91/1462) and 10.3% (150/1451) in the study and control groups, respectively. Overall, patients receiving JAK inhibitors had a lower 28-day mortality rate than those in the control group (RR, 0.60; 95% CI, 0.47–0.77; I2 = 0%, Figure 3). The results remained unchanged after the leave-one-out sensitivity test was conducted. Additionally, the pooled analysis of 2 phase 2 trials [14,18] and 3 phase 3 trials [15–17] revealed similar findings (phase 2 trial: RR, 0.15; 95% CI, 0.03–0.90; I2 = 0%; phase 3 trial: RR, 0.62; 95% CI, 0.48–0.80; I2 = 0%). The subgroup group analysis of 4 RCTs [14–17] that compared those receiving oral JAK inhibitors with those in the control group also revealed that the study group had a lower mortality rate than the control group (RR, 0.51; 95% CI, 0.48–0.79; I2 = 0%). Moreover, patients receiving baricitinib had a lower mortality rate than those in the control group (RR, 0.63; 95% CI, 0.48–0.81; I2 = 0%) in the subgroup analysis of 2 RCTs [16,17]. All of these findings were based on the analysis of the included RCTs with low heterogeneity.
Figure 3.
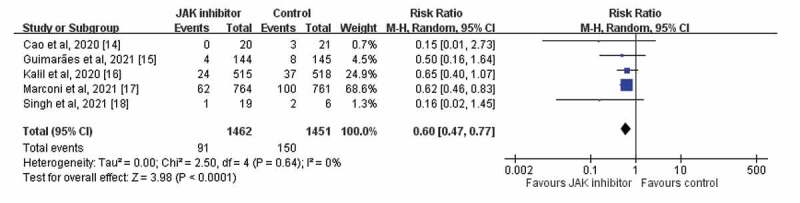
Forest plot of effect of Janus kinase (JAK) inhibitors compared with controls for the risk of 28-day mortality
3.4. Secondary outcomes
Patients receiving JAK inhibitors also had a lower 14-day mortality rate than those in the control group (RR, 0.60; 95% CI, 0.42–0.85; I2 = 0%). This finding remained consistent in the sensitivity test and in the subgroup analysis, according to the study design (phase 2 or phase 3), study group using oral JAK inhibitors, and patients using baricitinib. Moreover, the study group was associated with a higher rate of clinical improvement than was the control group (RR, 1.05; 95% CI, 1.02–1.09; I2 = 0%). Compared with the control group, the study group required less MV or ECMO (RR, 0.64; 95% CI, 0.50–0.84; I2 = 0%).
The study group had a similar risk of AEs as the control group did (RR, 0.94; 95% CI, 0.82–1.08; I2 = 46%, Figure 4) in the pooled analysis of 5 RCTs [14–18]. In the pooled analysis of 4 RCTs [14–17] with available data regarding serious AEs, the study group exhibited a lower risk of serious AEs than the control group did (RR, 0.81; 95% CI, 0.67–0.99; I2 = 14%). The 2 studies [15,17] that reported the risk of discontinuation of the study drug indicated no significant difference in AEs between the study and control groups (RR, 1.48; 95% CI, 0.38–5.72; I2 = 86%). The study group exhibited a similar risk of infection (RR, 0.96; 95% CI, 0.76–1.20; I2 = 0%), sepsis (RR, 0.77; 95% CI, 0.50–1.19; I2 = 9%), and septic shock (RR, 0.61; 95% CI, 0.36–1.04; I2 = 0%; Figure 5) as the control group.
Figure 4.
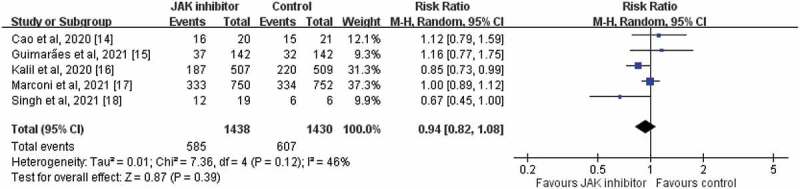
Forest plot of effect of JAK inhibitors compared with controls for the risk of any adverse events
Figure 5.
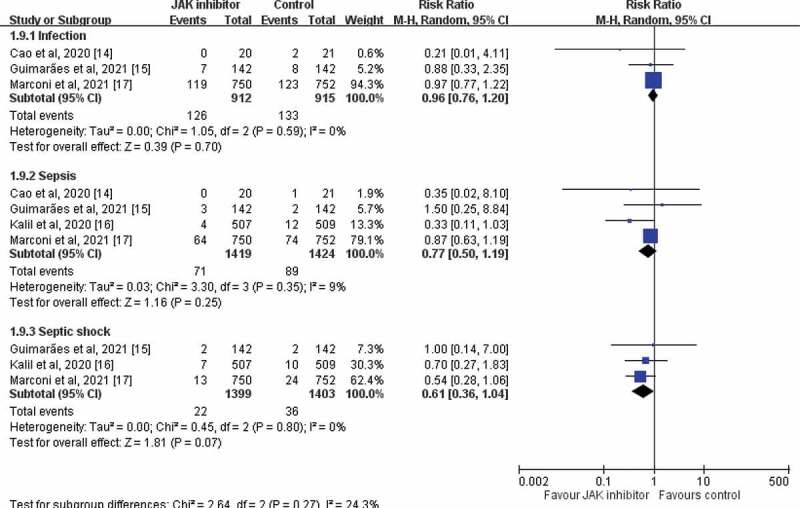
Forest plot of effect of JAK inhibitors compared with controls for the risk of infection
4. Discussion
In this meta-analysis, 5 RCTs [14–18] were reviewed to investigate the clinical efficacy and safety of using JAK inhibitors, namely oral baricitinib, tofacitinib, ruxolitinib, and inhaled nezulcitinib, to treat hospitalized patients with COVID-19. Patients who received JAK inhibitors had a more favorable clinical outcome than the control group in several areas. First, the overall 28-day mortality rate of the patients with COVID-19 receiving JAK inhibitors was significantly lower than that of the control group. Second, this lower mortality rate remained unchanged in the leave-one-out sensitivity analysis and various subgroup analyses. Third, the JAK inhibitors group had a lower 14-day mortality rate, a higher rate of clinical improvement, and less need of MV or ECMO than the control group. In summary, JAK inhibitors are effective anti-inflammatory agents that can improve the clinical outcomes of hospitalized patients with COVID-19, and they have a potential role in the treatment of this population during the COVID-19 pandemic, in which the effective agent against SARS-CoV-2 infections is limited. These findings are consistent with previous meta-analyses [20,22] that included only 1400 patients. However, our analysis was based on 5 RCTs with 2913 patients, and thus further confirms the effectiveness of JAK inhibitors.
In addition to clinical efficacy, we also evaluated safety. We found that adding JAK inhibitors did not result in a higher risk of AEs among participants in the study group than among those in the control group. Moreover, the risks of infection, sepsis, and septic shock were similar between the JAK inhibitors and control groups. These findings suggest that JAK inhibitors do not present additional risks and are thus recommended for the treatment of patients with COVID-19.
This meta-analysis has several strengths. First, only RCTs were included; thus, the evidence level of this study is potentially stronger than previous meta-analyses that included mainly observational studies [19,21]. Second, in contrast to previous meta-analyses [20,22] of RCTs that included 3 or 4 RCTs, our study included 5 RCTs with many more patients and could thus provide more information.
However, this study also has some limitations. First, the number of studies and patients for each JAK inhibitor – baricitinib, tofacitinib, ruxolitinib, and nezulcitinib – were limited. More large-scale RCTs are required to assess the effectiveness of each inhibitor. Second, this study focused on the clinical outcomes; therefore, the virological outcomes or change of inflammatory parameters were not evaluated. Third, one early-phase 2 clinical trial [18] had the deficit of the small size of this and consequent baseline differences in clinical characteristics, which could be associated with allocation bias. Another phase 2 study [14] had a small sample size of only 41, so their findings should be interpreted cautiously. Finally, this meta-analysis including 4 kinds of JAK inhibitors, – baricitinib, tofacitinib, ruxolitinib, and nezulcitinib, which can selectively inhibit different JAKs. Baricitinib and ruxolitinib are selective inhibitor of JAK1 and JAK2 but tofacitinib is the selective inhibitor of JAK 1 and JAK3, with functional selectivity for JAK2. In contrast, nezulcitinib was designed to target all JAK isoforms including JAK1, JAK2, and JAK3. Therefore, the clinical efficacy of each JAK inhibitor could vary and needs more RCTs to demonstrate the usefulness of each JAK inhibitor.
5. Conclusion
In conclusion, JAK inhibitors can help reduce the mortality rate and improve the clinical outcomes of hospitalized patients with COVID-19. Additionally, JAK inhibitors can be used safely in this clinical entity.
Appendix A. Search strategy
| PubMed search strategy – last searched on 7 September 2021 | Results | |
|---|---|---|
| 1 | Search: (((((((((Ruxolitinib[Title/Abstract]) OR (Tofacitinib[Title/Abstract])) OR (Oclacitinib[Title/Abstract])) OR (Baricitinib[Title/Abstract])) OR (Peficitinib[Title/Abstract])) OR (Fedratinib[Title/Abstract])) OR (Upadacitinib[Title/Abstract])) OR (Filgotinib[Title/Abstract])) OR (Delgocitinib[Title/Abstract])) OR (Nezulcitinib[Title/Abstract]) | 4058 |
| 2 | Search: ((((Covid-19[Title/Abstract]) OR (SARS-CoV-2[Title/Abstract])) OR (coronaviru[Title/Abstract])) OR (2019-nCoV[Title/Abstract])) OR (corona-virus[Title/Abstract]) | 162246 |
| 3 | Search: ((((((((((Ruxolitinib[Title/Abstract]) OR (Tofacitinib[Title/Abstract])) OR (Oclacitinib[Title/Abstract])) OR (Baricitinib[Title/Abstract])) OR (Peficitinib[Title/Abstract])) OR (Fedratinib[Title/Abstract])) OR (Upadacitinib[Title/Abstract])) OR (Filgotinib[Title/Abstract])) OR (Delgocitinib[Title/Abstract])) OR (Nezulcitinib[Title/Abstract])) AND (((((Covid-19[Title/Abstract]) OR (SARS-CoV-2[Title/Abstract])) OR (coronaviru[Title/Abstract])) OR (2019-nCoV[Title/Abstract])) OR (corona-virus[Title/Abstract])) | 232 |
| Web of Science search strategy – last searched on 7 September 2021 | Results | |
|---|---|---|
| 1 | Ruxolitinib (TOPIC) or Tofacitinib (TOPIC) or Oclacitinib (TOPIC) or Baricitinib (TOPIC) or Peficitinib (TOPIC) or Fedratinib (TOPIC) or Upadacitinib (TOPIC) or Filgotinib (TOPIC) or Delgocitinib (TOPIC) or Nezulcitinib (TOPIC) | 2346 |
| 2 | Covid-19 (TOPIC) or SARS-CoV-2 (TOPIC) or coronavirus (TOPIC) or 2019-nCoV (TOPIC) or corona-virus (TOPIC) | 139231 |
| 3 | #1 AND #2 | 202 |
| Ovid medline search strategy – last searched on 7 September 2021 | Results | |
|---|---|---|
| 1 | (Ruxolitinib or Tofacitinib or Oclacitinib or Baricitinib or Peficitinib or Fedratinib or Upadacitinib or Filgotinib or Delgocitinib or Nezulcitinib).ab. | 2950 |
| 2 | (Covid-19 or SARS-CoV-2 or coronavirus or 2019-nCoV or corona-virus).ab. | 107616 |
| 3 | 1 and 2 | 144 |
| Cochrane Library search strategy – last searched on 7 September 2021 | ||
|---|---|---|
| 1 | (Ruxolitinib):ti,ab,kw OR (Tofacitinib):ti,ab,kw OR (Oclacitinib):ti,ab,kw OR (Baricitinib):ti,ab,kw OR (Peficitinib):ti,ab,kw OR (Fedratinib):ti,ab,kw OR (Upadacitinib):ti,ab,kw OR (Filgotinib):ti,ab,kw OR (Delgocitinib):ti,ab,kw OR (Nezulcitinib):ti,ab,kw | 2443 |
| 2 | (Covid 19):ti,ab,kw OR (SARS CoV 2):ti,ab,kw OR (coronavirus):ti,ab,kw OR (2019 nCoV):ti,ab,kw OR (corona virus):ti,ab,kw | 7371 |
| 3 | #1 AND #2 | 60 |
Declaration of interests
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
Conception: SHH and WTL
Study design: SHL, CKW, SPC
Analysis and interpretation: SHL, CKW, LCL
Drafted or written: CKW and SHH
Substantially revised or critically review: SHH and WTL
All authors have agreed on the journal to which the article will be submitted and reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage. In addition, all authors agree to take responsibility and be accountable for the contents of the article and to share responsibility to resolve any questions raised about the accuracy or integrity of the published work.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.WHO . [cited 2021. Sept 7]. Available from: https://covid19.who.int/
- 2.Lai CC, Liu YH, Wang CY, et al. Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): facts and myths. J Microbiol Immunol Infect. 2020;53(3):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shankar-Hari M, Vale CL, Godolphin PJ, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. Jama. 2021;326(6):499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sterne JAC, Murthy S, Diaz JV, et al. Association between administration of systemic corticosteroids and mortality among critically Ill patients with COVID-19: a meta-analysis. Jama. 2020;324(13):1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NIH . [cited 2021. Sept 7]. Available from: https://www.covid19treatmentguidelines.nih.gov/about-the-guidelines/whats-new/
- 6.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis Campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: first update. Crit Care Med. 2021;49(3):e219–e234. [DOI] [PubMed] [Google Scholar]
- 7.Fajgenbaum DC, June CH.. Cytokine Storm. N Engl J Med. 2020;383(23):2255–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383(25):2451–2460. [DOI] [PubMed] [Google Scholar]
- 9.Dörner T, Tanaka Y, Petri MA, et al. Baricitinib-associated changes in global gene expression during a 24-week phase II clinical systemic lupus erythematosus trial implicates a mechanism of action through multiple immune-related pathways. Lupus Sci Med. 2020;7(1):e000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka Y, Emoto K, Cai Z, et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol. 2016;43(3):504–511. [DOI] [PubMed] [Google Scholar]
- 11.Chapman S, Gold LS, and Lim HW. Janus kinase inhibitors in dermatology: part II. A comprehensive review. J Am Acad Dermatol. 2021:S0190-9622(21)02022-3 doi: 10.1016/j.jaad.2021.06.873 . [DOI] [PubMed] [Google Scholar]
- 12.You H, Xu D, Zhao J, et al. JAK inhibitors: prospects in connective tissue diseases. Clin Rev Allergy Immunol. 2020;59(3):334–351. [DOI] [PubMed] [Google Scholar]
- 13.Gatti M, Turrini E, Raschi E, et al. Janus kinase inhibitors and Coronavirus disease (COVID)-19: rationale, clinical evidence and safety issues. Pharmaceuticals (Basel). 2021;14(8):738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol. 2020;146(1):137–146.e133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N Engl J Med. 2021;385(5). DOI: 10.1056/NEJMoa2101643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384(9):795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A large RCT assessed the efficacy of bariciinib pluse remdesivir for hospitalized patients with COVID-19.
- 17.Marconi VC, Ramanan AV, de Bono S, et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021. DOI: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• A large RCT evaluated the usefulness of for the treatment of hospitalised adults with COVID-19.
- 18.Singh D, Bogus M, Moskalenko V, et al. A phase 2 multiple ascending dose study of the inhaled pan-JAK inhibitor nezulcitinib (TD-0903) in severe COVID-19. Eur Respir J. 2021;58(4):2100673. [DOI] [PMC free article] [PubMed] [Google Scholar]; • An early phase 2 trial of inhaled JAK inhibitor for patients with COVID-19.
- 19.Chen CX, Wang JJ, Li H, et al. JAK-inhibitors for coronavirus disease-2019 (COVID-19): a meta-analysis. Leukemia. 2021;1–5. DOI: 10.1038/s41375-020-0954-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CY, Chen WC, Hsu CK, et al. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Int Immunopharmacol. 2021;99:108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wijaya I, Andhika R, Huang I, et al. The use of Janus Kinase inhibitors in hospitalized patients with COVID-19: systematic review and meta-analysis. Clin Epidemiol Glob Health. 2021;11:100755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patoulias D, Doumas M, Papadopoulos C, et al. Janus kinase inhibitors and major COVID-19 outcomes: time to forget the two faces of Janus! A meta-analysis of randomized controlled trials. Clin Rheumatol. 2021;1–4. DOI: 10.1007/s10067-020-05517-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Page MJ, McKenzie JE, Bossuyt PM, et al. Updating guidance for reporting systematic reviews: development of the PRISMA 2020 statement. J Clin Epidemiol. 2021;134:103–112. [DOI] [PubMed] [Google Scholar]
- 24.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]


