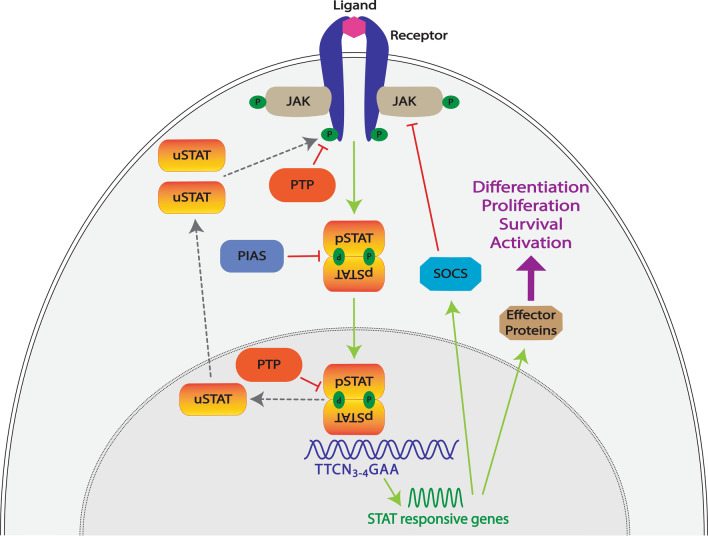
Fig. 2.
Canonical STAT mode of action. Schematic representation of the archetypal ‘canonical’ STAT functional modality and its control. In this paradigm, STAT proteins (orange/yellow) exist in the cytoplasm as latent, unphosphorylated STAT (uSTAT) molecules. In response to binding of their cognate extracellular ligands (light pink), transmembrane receptors (dark blue) undergo conformational changes that results in the activation of kinases such as the receptor-associated JAKs (light brown), which subsequently mediate phosphorylation (P, green) of tyrosine residues within the intracellular receptor complex, thereby creating docking sites for signaling molecules, including uSTATs. These in turn become tyrosine phosphorylated, with the phosphorylated (pSTAT) molecules able to form dimers that can translocate into the nucleus and bind to specific DNA sequences (blue) to activate the transcription of responsive genes. These encode effector proteins (brown) responsible for cell differentiation, proliferation, survival and activation, as well as SOCS proteins (blue). These mediate a negative feedback loop by blocking STAT activation through interfering with STAT docking, inhibiting JAKs and/or mediating degradation of receptor signaling components. Other negative regulators include PIAS proteins (grey blue) that act via blocking STAT dimerization and nuclear entry and Protein tyrosine phosphatase (PTP) proteins (orange) that can dephosphorylate receptor complex components in the cytoplasm as well as pSTAT molecules in the nucleus to regenerate uSTAT molecules that return to the cytoplasm