Fig. 5: Tmem97 is a selective protein target of probe 2.
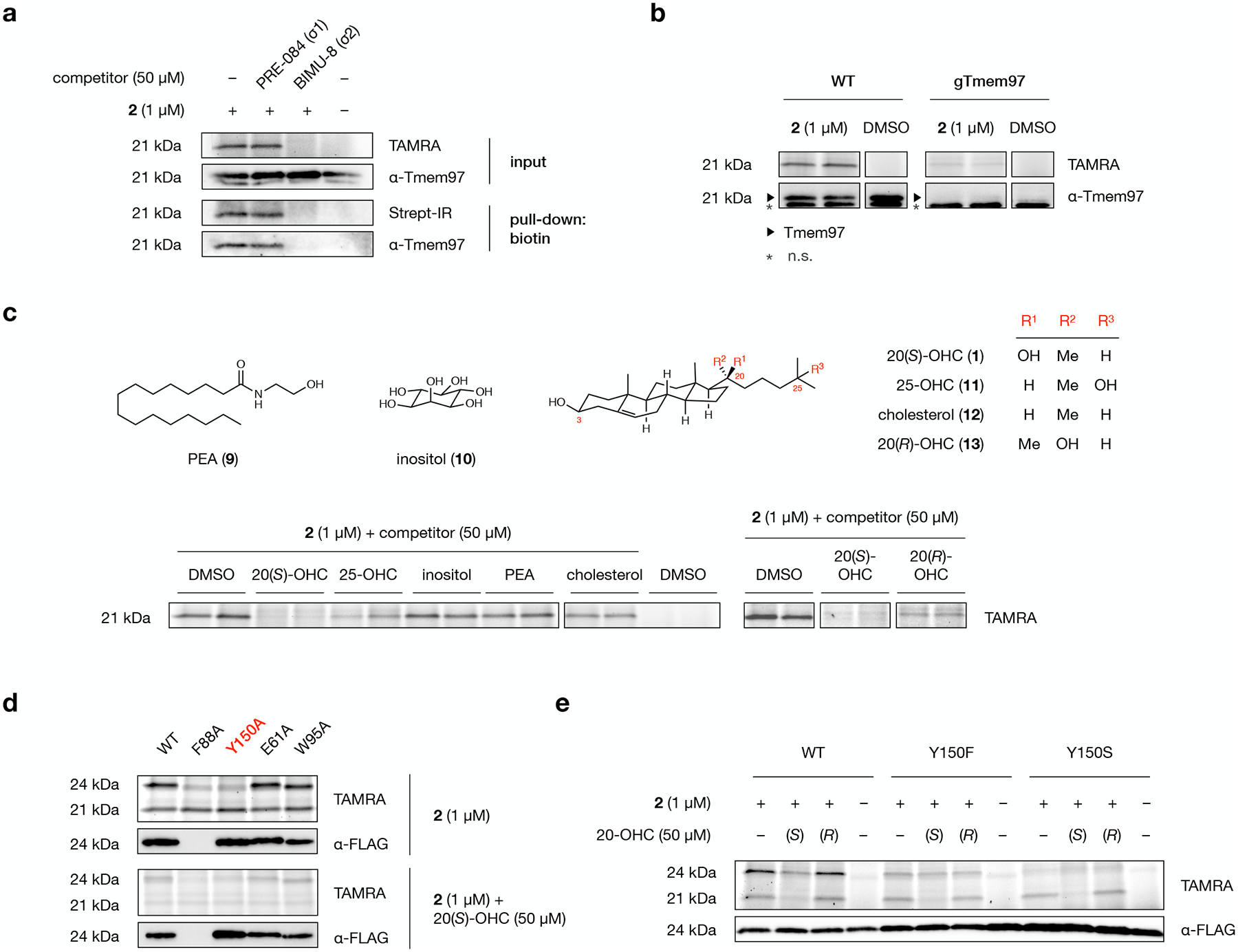
a, A TAMRA-labeled band at 21 kDa in the membrane proteome of 2-labeled NIH-3T3 cells is eliminated by competition with BIMU-8, a selective Tmem97 (σ2 receptor) ligand, but not PRE-084, a selective σ1 receptor ligand. Likewise, competition with BIMU-8, but not PRE-084, reduces labeling, biotin-streptavidin enrichment, and detection of the band at 21 kDa by a Tmem97 antibody.
b, TAMRA fluorescence and Tmem97 antibody signal from wild-type and Tmem97-KO NIH-3T3 cells treated with 2 (duplicate samples) or DMSO. An asterisk indicates non-specific (n.s.) protein detection by the Tmem97 antibody.
c, Representative in-gel analysis of probe 2 labeling at 21 kDa in the presence of 20(S)-OHC (1), 25-OHC (11), inositol (10), palmitoylethanolamine (PEA, 9), cholesterol (12), and 20(R)-OHC (13) as competitors in NIH-3T3 cells. For quantification, see Extended Data Fig. 5c.
d, Representative in-gel analysis of probe 2 labeling of Tmem97 mutants expressed as Tmem97-Myc-FLAG fusion constructs in HEK293T cells. Alanine substitution of the Y150 residue in the Tmem97:20(S)-OHC binding site eliminates labeling of overexpressed Tmem97. E61A and W95A mutants remain susceptible to labeling by 2, while an F88A mutant fails to express in cells.
e, Representative in-gel analysis of probe 2 labeling of wild-type Tmem97, Tmem97(Y150F), or Tmem97(Y150S) in expressed as Tmem97-Myc-FLAG fusion constructs in HEK293T cells. A Y150F mutation decreases labeling efficiency, reduces competition by 20(S)-OHC, and eliminates stereoselectivity for 20(S)- over 20(R)-OHC. A Y150S mutation eliminates probe labeling. For quantification, see Extended Data Fig. 6b.
Experiments in a, b, and d were repeated two times independently with similar results. In d and e, endogenous Tmem97 appears at 21 kDa; overexpressed Tmem97-Myc-FLAG appears at 24 kDa due to the epitope tags.
