Extended Data Figure 6. Assessment of ligand density, of the presence of all-trans retinal, and of the conformational heterogeneity of the GRK1 RH domain.
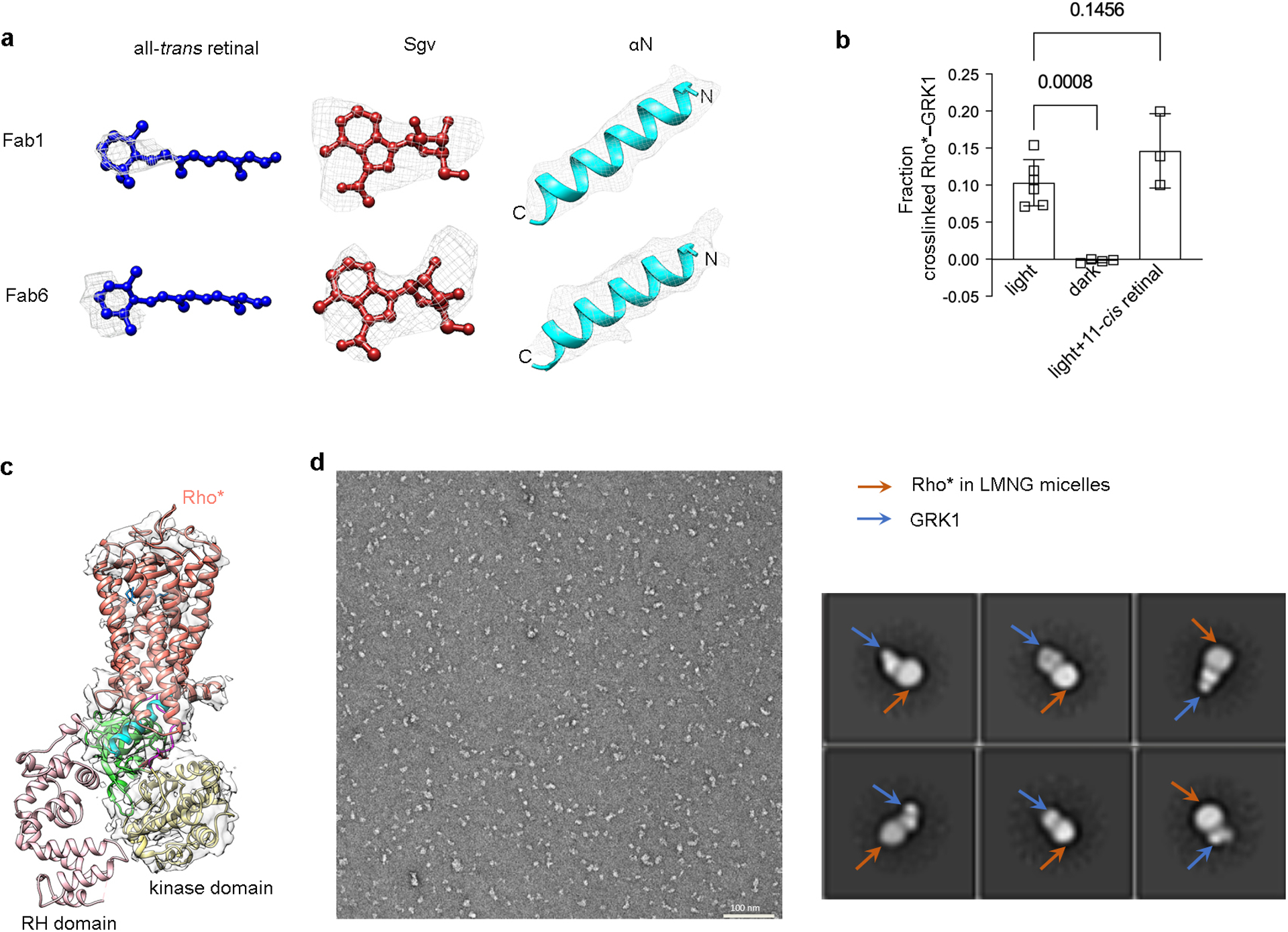
a) Electron density of all trans retinal, contoured at 17 σ (Fab1 map) and 13 σ (Fab6 map); Sgv, contoured at 24 σ (Fab1 map) and 26 σ (Fab6 map); αN, contoured at 21 σ (Fab1 map) and 25 σ (Fab6 map). c) Light and GRK ligand dependence of the crosslinking reaction between rhodopsin and GRK1. 11-cis retinal undergoes isomerization upon light exposure to all-trans retinal, which serves as a full agonist for rhodopsin. The crosslinking level of GRK1 with rhodopsin in the dark (n=4) and in the light with excess 11-cis retinal (n=3) were compared with that of GRK1 with rhodopsin in the light using one-way ANOVA followed by a Dunnett’s multiple comparison test. Symbols represent the mean value ± standard deviation. d) Density for the RH domain is not observed by cryo-EM in all our reconstructions. The map of Rho*–GRK1S5E/EE–Fab1 is shown here as an example. e) A representative negative stain EM micrograph of Rho*–GRK1 complex solubilized in LMNG (left) along with representative 2D averages (right), indicating heterogeneity in the bound GRK1. The smaller, variably positioned domain is interpreted as the RH domain.
