Figure 4. CLMS confirms the cryo-EM structure and reveals dynamics in the receptor C-terminal tail.
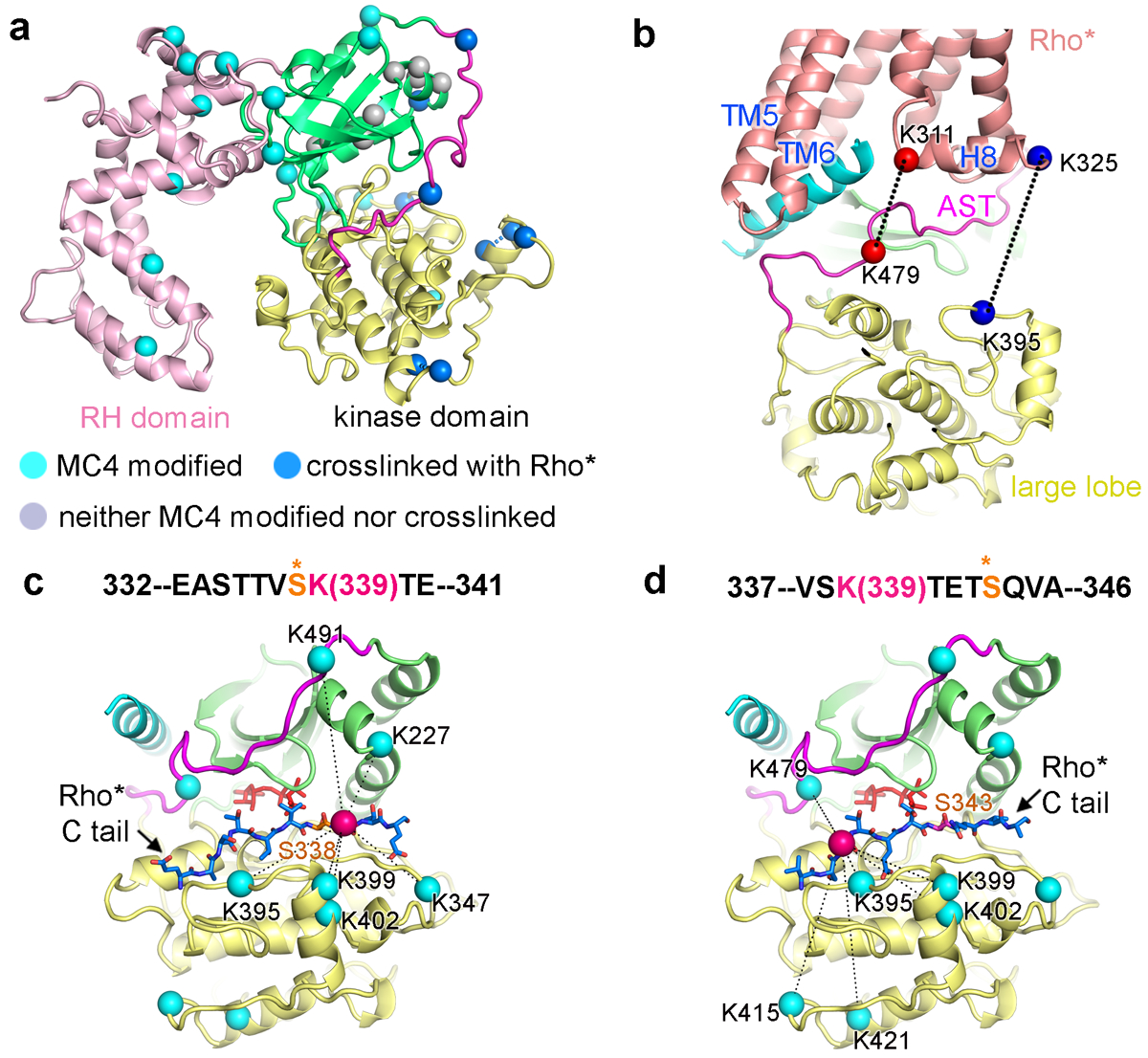
a) Lysine residues of GRK1 that crosslink with Rho* are indicated by blue Cα atoms, those that are dead-end modified are colored cyan (suggesting solvent accessibility), and those that are unreactive are colored grey (suggesting protection). b) Two prominent pairs of crosslinked lysines between Rho* and the kinase domain of GRK1 are highlighted with spheres connected by dashed lines. c, d) The C terminus of Rho* was modeled bounded to the peptide binding channel of GRK1 based on PDB entry 1O6L42. Rho*-Lys339 Cα is drawn as a red sphere, whereas the Cα atoms of lysines on GRK1 that crosslink with it are cyan. ATP is colored orange. c) Rho*-Ser338 in the phosphoacceptor site. d) Rho*-Ser343 in the phosphoacceptor site.
