Figure 5. A generalized model for GRK activation by anionic lipids and activated GPCRs.
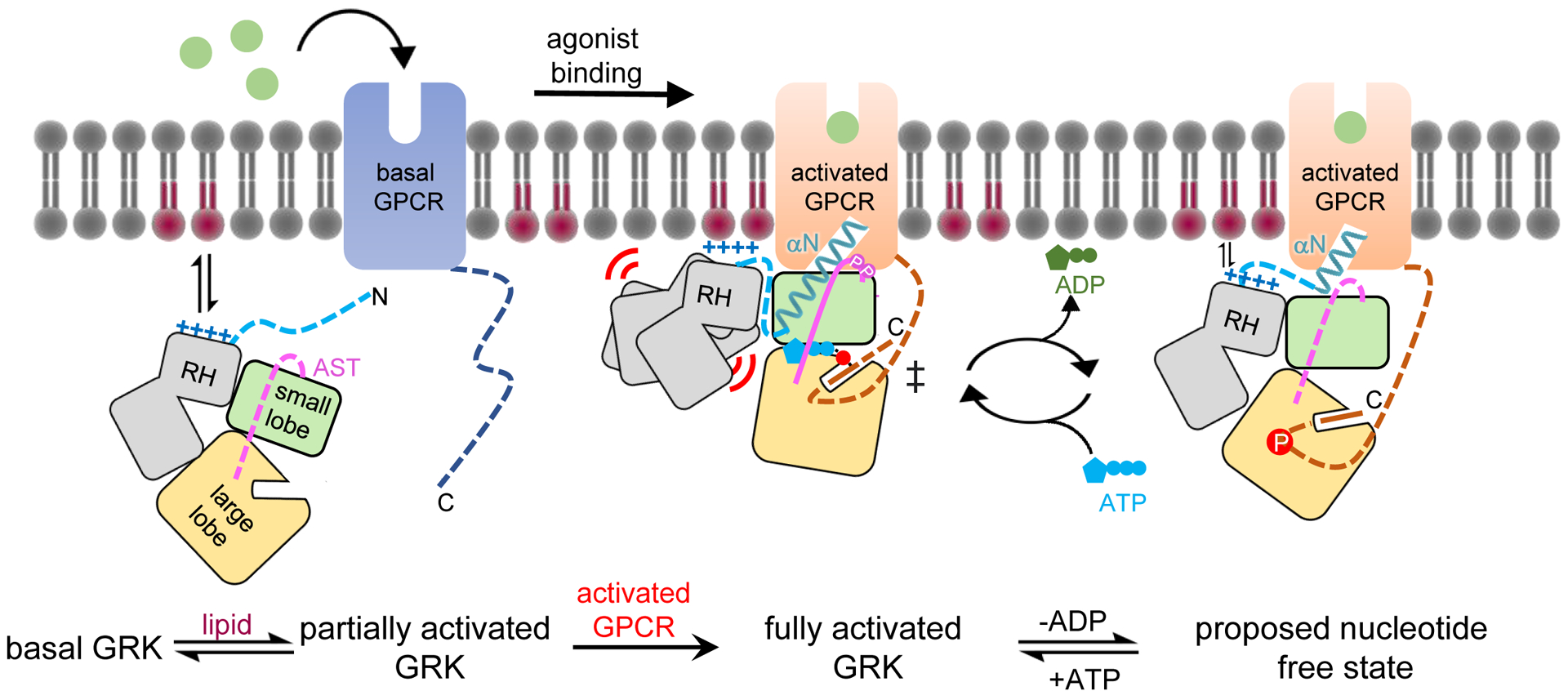
In its basal state, the GRK kinase domain is in an open conformation with its small and large lobes engaged with both lobes of the RH domain. The N terminus and portions of the AST in the GRK are disordered. A basic region adjacent to the receptor interface (α0 helix region in GRK1) promotes membrane association. Upon receptor activation, the GRK N terminus forms a helix (αN) that docks into the cytoplasmic cleft of the activated GPCR. αN also packs against the small lobe and AST to allosterically trigger kinase domain closure, aligning ATP with the phosphoacceptor in the C tail of GPCR in a transition state-like complex. GRKs may need to at least partially dissociate from the receptor to efficiently exchange adenine nucleotides, and in this state αN and the large lobe could remain bound to the cytoplasmic cleft and the phosphorylated tail of the receptor, respectively, to facilitate additional rounds of phosphorylation. In GRK1, and perhaps in the closely related GRK4 subfamily of GRKs, receptor binding also induces conformation changes that displace the RH domain. This could serve to strengthen membrane interactions mediated by the RH domain and its associated loops, including the α0 helix. GRK2 and GRK3 lack an analogous α0 helix and there is evidence that their RH domains may not be as dynamic in receptor complexes. Pink and red circles indicate GRK autophosphorylation sites and Rho* phosphosites, respectively.
