Supplemental Digital Content is available in the text.
Key Words: bone mineral density, MRI, cognitive function, executive function, visual memory, verbal memory
Background:
Bone mineral density (BMD) is a potential surrogate marker of lifetime estrogen exposure previously linked to increased risk of Alzheimer dementia among elderly women. We examine the association between BMD in the “young old” with imaging biomarkers of brain aging and cognitive performance.
Methods:
Offspring participants (N=1905, mean age 66) of a population-based cohort who had BMD, brain imaging and detailed cognitive assessment were included in the study. Sex-stratified, linear, and logistic regression models were used for analysis.
Results:
Higher femoral neck BMD was associated with lower white matter hyperintensity burden and better performance on Trails B-A in both sexes, even after adjustment for cerebrovascular risk factors. Among women, the positive association with Trails B-A performance was seen only in APOE4 allele carriers. Higher BMD measurements were linked to better visual reproductions test performance in men. Finally, among women, higher femoral trochanter BMD was associated with better logical memory and Hooper visual organization test performance.
Conclusion:
Among the “young old,” higher BMD is associated with less white matter hyperintensity burden and better, domain-specific, cognitive performance. This suggests that lifetime estrogen exposure may modulate the degree of cumulative vascular brain injury independent of cerebrovascular risk factors.
Osteoporosis and fragility fractures have a high prevalence among persons with dementia and greatly contribute to the morbidity and mortality of an aging population.1–3 In addition to dietary and mobility factors that contribute to low bone mineral density (BMD) in older age,1 there appears to be a common pathway underlying neurodegeneration and bone health as low BMD has been associated with more advanced normal age-related brain parenchyma atrophy,4 prevalent Alzheimer disease (AD) and vascular dementia,5,6 as well as an increased risk for developing AD.7,8 One putative link between BMD and brain aging is cumulative exposure to estrogen supported by data showing that the length of the reproductive period in women was inversely related to their risk of cognitive impairment and protective against the development of AD.9 Other factors that may shape this association include atherosclerosis,10 physical activity,11,12 and APOE4 allele status,13 all of which have been linked to both bone and brain health. We now expand previous work done in the Framingham Heart Study (FHS) Original Cohort (mean age, 77 y)7 to the offspring (mean age, 66 y) in order to assess the association between BMD and neuroimaging biomarkers of brain aging and performance in specific cognitive domains among younger, nondemented individuals. We hypothesize that low BMD—a surrogate marker of lifetime estrogen exposure—may be an indicator of poor brain health even among the “young old” independent of other risk factors.
METHODS
Study Design
The Framingham study is an ongoing longitudinal, community-based cohort study that began in 1948 with the enrollment of 5209 participants into the Original Cohort to prospectively investigate risk factors for cardiovascular disease (CVD).
Study population in 1971, the offspring of the Original Cohort and their spouses (n=5124) were enrolled in the Framingham Offspring Study (Gen 2) and have attended clinic examinations approximately every 4 years since. Offspring participants who took part in examination cycles 7.5 and 8 (n=2025) were invited to attend an osteoporosis examination and to undergo brain magnetic resonance imaging (MRI) and a neuropsychological (NP) battery. After exclusion for established clinical dementia, stroke, or other neurological conditions that could affect MRI measurements (tumor, multiple sclerosis, significant traumatic brain injury, etc.) 1905 participants in Gen 2 had both BMD measurements and NP testing available and of these 1599 participants also had MRI brain performed (Fig. 1).
FIGURE 1.
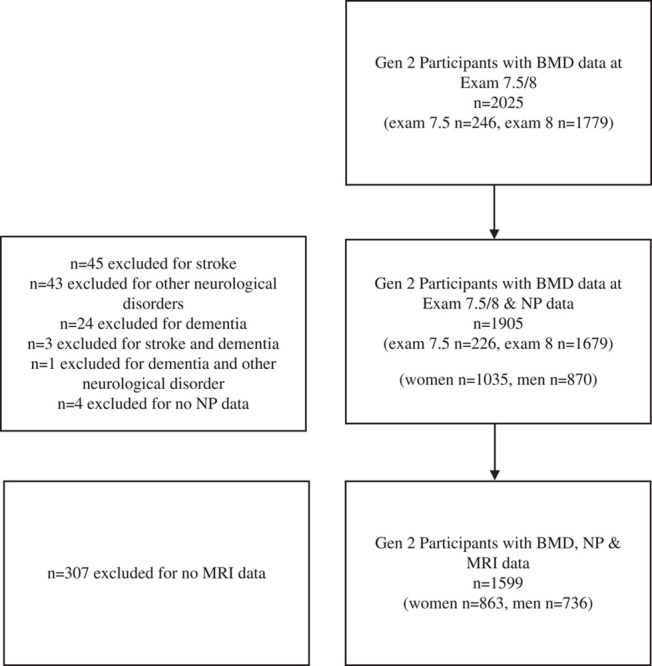
Gen 2 study participants. BMD indicates bone mineral density; MRI, magnetic resonance imaging; NP, neuropsychological.
BMD Measurements
BMD of the hip was measured using dual-energy X-ray absorptiometry (DXA) with a GE Lunar Prodigy fan-beam densitometer (GE Healthcare Inc., GE Healthcare, Piscataway, NJ), using standard positioning recommended by the manufacturer with BMD measured in g/cm2. The right hip was scanned unless there was a history of previous fracture or hip replacement, in which case the left side was scanned. Measurements for femoral neck (FN), and femoral trochanter (FT) BMD were included. The coefficients of variation for FN and FT were 1.7% and 2.5%, respectively.
NP Testing
A battery of NP tests to measure aspects of cognitive function associated with brain aging were administered using standard administration protocols by trained examiners. Details of these tests administered in the FHS cohorts have been published previously.14,15
Executive function and processing speed were assessed based on time to complete Trail Making B-A (Trails B-A). Verbal and visual memory was assessed with the use of the delayed recall components of Logical Memory (LM-d) and Visual Reproductions (VR-d) tests, respectively. The test of similarities from the Wechsler Adult Intelligence Scale (WAIS) involves asking participants to indicate in which way 2 items are alike to test abstraction, reasoning, verbal comprehension, and categorization. Finally, the Hooper Visual Organization Test (HVOT) measures visuoperceptual skills. Trails B-A and HVOT were natural log-transformed to normalize their skewed distributions. We reversed the sign of Trails B-A so that higher scores indicate superior performance for all cognitive measures.
Methodology for Volumetric Brain MRI
Details of brain MRI acquisition parameters, blinded analysis, definition of brain volumes, and normative data for the FHS Gen 2 cohort has been published previously.16–20 Three MRI measures of subclinical brain aging were used as primary outcomes: total cerebral brain volume, a measure of overall (AD-type and vascular) brain aging, hippocampal volume (HV) which in older adults has been associated to AD-type pathology was manually defined as described earlier.21 White matter hyperintensities volume (WMHV), a measure of vascular brain injury, was determined according to previously published methods16,21 Each of these was analyzed as % of total cranial volume to correct for differences in head size.17 WMHV was log-transformed to normalize its distribution. All analyses were performed using a custom-designed image analysis package, QUANTA 6.2, operating on a Sun Microsystems (Santa Clara, CA) Ultra 5 work station.
Vascular Risk Factors
Systolic blood pressure was calculated as the average of 2 seated resting measurements performed by a physician. Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL, a random blood glucose ≥200 mg/dL, previous diagnosis of diabetes mellitus or being on hypoglycemic medications or insulin. Fasting plasma TC and TG were measured enzymatically according to Centers for Disease Control and Prevention standards. Current cigarette smoking was defined as self-reported regular use in the year before the targeted examination cycle. In addition to the previously mentioned risk factors, history of CVD, as defined by history of prior coronary artery disease, congestive heart failure of peripheral vascular disease, as well as history of atrial fibrillation were also used in the analysis.
Other Variables
Physical activity index (PAI) is a composite score constructed for each participant by weighting each hour in a participant’s typical day based on their activity level [sleep weighting factor (WF)=1, sedentary WF=1.1, slight activity WF=1.5, moderate activity WF=2.4, and heavy activity WF=5] and summing up these weighted hours over a 24-hour period. PAI has been shown to have fair correlation to accelerometer-determined physical activity measures and lower PAI is linked to a higher risk of incident dementia.12 BMI was calculated as weight divided by height2 (kg/m2). Osteoporosis medications was based on World Health Organization (WHO) Anatomical Therapeutic Chemical (ATC) codes and included reported active use of biphosphonates, calcium and its combinations with vitamin D and/or other drugs, parathyroid hormones and analogs, antiparathyroid agents, hormonal contraceptives for systemic use, and estrogen use. Participants who had BMD measured at exam 7.5 reported use of antidepressant medication as 0: no, 1: yes-now, 2: yes-not now, 3: maybe and 4: unknown. For our analysis, only code 1 was considered as active use of antidepressants. Antidepressant use in exam 8 was based on WHO ATC codes (current use of nonselective monoamine reuptake inhibitors, selective serotonin reuptake inhibitors, monoamine oxidase inhibitors, nonselective, monoamine oxidase a inhibitors, and other antidepressants).
Statistical Analysis
Sex and age are strong determinants of BMD. In the analysis, we used sex-stratified linear (for continuous variables) and logistic (for binary variables) regression models to examine the cross-sectional association between BMD measurements at each site and MRI phenotypes, as well as between BMD measurements and performance per cognitive domain.
In primary analysis (Model 1), for the MRI measurements we adjusted for age, age-squared and time between the participant exam and the time of MRI acquisition. For the cognitive analysis, we adjusted for age, educational status and time between the participant exam and the time of NP assessment. We also investigated potential effect modification by APOE genotype by including interaction terms in the model. Where evidence of effect modification was seen, we stratified by APOE. In secondary analysis (Model 2), we additionally adjusted for the vascular risk factors described above, which are strongly associated with both outcome measures in our study. In sensitivity analysis, we examined the individual effect of variables primarily affecting BMD, namely PAI,22 BMI23 and use of osteoporosis and antidepressant medications24 on Model 2 in subgroups of participants with available data. We used log-transformations as necessary to normalize variables with skewed distributions.
All protocols were approved by the Boston University Medical Center Institutional Review Board, and participants provided written informed consent. All data collected on Framingham Study participants, including those used in our analysis, are available to qualified scientific investigators outside FHS who complete a research application, in accordance to FHS data sharing policies.
RESULTS
Baseline demographic and clinical characteristics are presented by sex in Table 1.
TABLE 1.
Baseline Demographic and Clinical Characteristics
| Gen 2 | |||
|---|---|---|---|
| Men (n=870) | Women (n=1035) | Significance | |
| Age, years | 66.28 (8.85) | 65.64 (9.17) | NS |
| Time interval between exam to NP/MRI evaluation, years | 0.51 (0.71) | 0.56 (0.78) | NS |
| Systolic blood pressure, mg/dL | 129.06 (16.68) | 127.51 (17.56) | NS |
| BMI, kg/m2 | 28.33 [25.81, 31.31] | 26.83 [23.65, 31.07] | * |
| Physical activity index | 35.57 (6.17) | 34.98 (5.10) | * |
| Total cholesterol, mg/dL | 175.70 (34.75) | 199.61 (35.96) | * |
| Triglycerides, mg/dL | 104 [75, 143] | 102 [73, 142] | NS |
| Education, n (%) | * | ||
| Up to high school degree | 242 (27.82) | 321 (31.01) | |
| Some college | 220 (25.29) | 365 (35.27) | |
| ≥College graduate | 408 (46.90) | 349 (33.72) | |
| Smoking, n (%) | 66 (7.59) | 101 (9.76) | NS |
| APOE4, n (%) | 192 (22.59) | 212 (20.99) | NS |
| Osteoporosis drug, n (%) | 44 (5.33) | 260 (25.92) | * |
| Antidepressant drug, n (%) | 69 (8.36) | 180 (17.93) | * |
| Lipid lowering drug, n (%) | 432 (49.66) | 406 (39.23) | * |
| Antihypertension drug, n (%) | 456 (52.47) | 433 (41.84) | * |
| Diabetes mellitus, n (%) | 160 (18.63) | 106 (10.44) | * |
| Prevalent CVD, n (%) | 139 (15.98) | 94 (9.08) | * |
| Prevalent AF, n (%) | 65 (7.47) | 39 (3.77) | * |
| Bone mineral density | |||
| Femoral neck BMD, g/cm2 | 0.96 (0.14) | 0.87 (0.13) | * |
| Femoral trochanter BMD, g/cm2 | 0.88 (0.14) | 0.71 (0.13) | * |
| Cognitive tests | |||
| LM-d recall score | 10.63 (3.81) | 11.65 (3.72) | * |
| VR-d recall score | 8.09 (3.33) | 8.1 (3.31) | NS |
| Trail making B-A, minutes | 0.36 [0.31, 0.39] | 0.36 [0.32, 0.40] | * |
| Similarities test score | 17.08 (3.80) | 16.79 (3.50) | NS |
| HVOT score | 25.0 [23.5, 27.0] | 26.0 [24.0, 27.0] | * |
| Brain MRI measures | |||
| TCBV, % of TCV | 75.97 (2.68) | 76.35 (2.50) | * |
| HPV, % of TCV | 0.53 (0.05) | 0.55 (0.05) | * |
| WMHV, % of TCV | 0.07 [0.04, 0.14] | 0.07 [0.04, 0.14] | NS |
AF indicates atrial fibrillation; APOE4, apolipoprotein E4 allele; BMD, bone mineral density; BMI, body mass index; CVD, cardiovascular disease; HPV, hippocampal volume; HVOT, Hooper Visual Organization test score; LM-d, logical memory delayed recall; MRI, magnetic resonance imaging; TCBV, total cerebral brain volume; TCV, total intracranial volume; VR-d, visual reproduction delayed; WMHV, white matter hyperintensity volume.
Data are mean (SD) or median [quartile 1 to quartile 3], unless stated otherwise.
Significant difference in characteristic between sexes (P<0.05).
Nonsignificant (P≥0.05).
BMD and Brain MRI Measures
Higher BMD measurement at the FN was associated with lower white matter hyperintensity (WMH) burden in both men and women. This association remained significant even after adjustment for vascular risk factors (Model 2) (Table 2). This association was not present when BMD was measured at the FT, except among women in Model 2 of the analysis. Among women, after adjustments in Model 2 for PAI, BMI, osteoporosis and antidepressant medications, the effect size remained largely the same, but the association lost significance (Supplemental Table B, Supplemental Digital Content 1, http://links.lww.com/WAD/A335). BMD measurements were not significantly associated with total cerebral brain volume or HV (Table 2).
TABLE 2.
MRI Outcomes
| Total Cerebral Brain Volume* | Hippocampal Volume* | White Matter Hyperintensity Volume* (log) | ||||
|---|---|---|---|---|---|---|
| BMD Measure | β [95% CI] | P | β [95% CI] | P | β [95% CI] | P |
| Men (M1 n=736, M2 n=724) | ||||||
| Femoral neck M1 | −0.23 [−1.26; 0.80] | 0.657 | −0.02 [−0.05; 0.004] | 0.098 | −0.70 [−1.23; −0.16] | 0.011 |
| Femoral neck M2 | −0.19 [−1.22; 0.84] | 0.715 | −0.02 [−0.05; 0.005] | 0.104 | −0.68 [−1.22; −0.13] | 0.015 |
| Femoral trochanter M1 | −0.08 [−1.07; 0.91] | 0.877 | −0.01 [−0.04; 0.01] | 0.277 | −0.49 [−1.01; 0.03] | 0.065 |
| Femoral trochanter M2 | −0.01 [−1.01; 0.99] | 0.988 | −0.02 [−0.04; 0.01] | 0.244 | −0.46 [−0.99; 0.07] | 0.091 |
| Women (M1 n=863, M2 n=847) | ||||||
| Femoral neck M1 | −0.43 [−1.50; 0.65] | 0.435 | 0.004 [−0.02; 0.03] | 0.748 | −0.59 [−1.16; −0.02] | 0.042 |
| Femoral neck M2 | −0.33 [−1.45; 0.79] | 0.562 | 0.01 [−0.02; 0.03] | 0.710 | −0.64 [−1.23; −0.04] | 0.035 |
| Femoral trochanter M1 | 0.52 [−0.51; 1.54] | 0.326 | 0.02 [−0.002; 0.05] | 0.076 | −0.49 [−1.04; 0.05] | 0.077 |
| Femoral trochanter M2 | 0.71 [−0.38; 1.80] | 0.200 | 0.02 [−0.004; 0.05] | 0.088 | −0.61 [−1.18; −0.03] | 0.040 |
M1: model 1 adjusted for age, age-squared, and time interval between exam and MRI assessment.
M2: model 2 adjusted for age, age-squared, time interval between exam and MRI assessment, systolic blood pressure, antihypertensive medication use, smoking, diabetes, prevalent CVD, atrial fibrillation, total cholesterol, and triglycerides.
Bold values indicate statistical significance (P<0.05).
% of total intracranial volume. β corresponds to the change in MRI assessment associated with a 1 unit-increase in BMD.
CI indicates confidence interval; CVD, cardiovascular disease; MRI, magnetic resonance imaging.
Effect of BMD on Cognitive Function
The effect of BMD on cognitive function differed among specific cognitive domains, BMD measurement site and between the sexes. Higher BMD measured at the FN was significantly associated with better performance on Trails B-A in both women and men and this association persisted after adjustment for vascular risk factors. In men, FN BMD was also positively associated with VR-d performance. In women, this association was seen with FT BMD, but the association was attenuated after adjusting for vascular risk factors. Finally, only among women, higher FT BMD was associated with better performance on LM-d and HVOT (model 1 only) (Table 3).
TABLE 3.
Neuropsychological Outcomes
| Logical Memory Delayed Recall | Visual Reproduction Delayed Recall | Trail Making B-A (Log) | Similarities Test | HVOT(Log) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BMD Measure | β [95% CI] | P | β [95% CI] | P | β [95% CI] | P | β [95% CI] | P | β [95% CI] | P |
| Men (M1 n=870, M2 n=857) | ||||||||||
| Femoral neck M1 | 1.31 [−0.43; 3.04] | 0.140 | 1.70 [0.23; 3.16] | 0.023 | 0.16 [0.05; 0.27] | 0.003 | 0.67 [−0.95; 2.30] | 0.417 | −0.01 [−0.24; 0.22] | 0.937 |
| Femoral neck M2 | 1.32 [−0.46; 3.09] | 0.145 | 1.64 [0.15; 3.12] | 0.031 | 0.16 [0.05; 0.27] | 0.004 | 0.89 [−0.77; 2.55] | 0.291 | −0.001 [−0.24; 0.24] | 0.996 |
| Femoral trochanter M1 | 0.71 [−0.98; 2.41] | 0.410 | 1.24 [−0.20; 2.68] | 0.091 | 0.15 [0.05; 0.26] | 0.004 | 0.10 [−1.49; 1.69] | 0.899 | −0.05 [−0.28; 0.17] | 0.645 |
| Femoral trochanter M2 | 0.69 [−1.07; 2.44] | 0.442 | 1.19 [−0.28; 2.67] | 0.112 | 0.14 [0.04; 0.25] | 0.008 | 0.24 [−1.4; 1.88] | 0.774 | −0.04 [−0.27; 0.20] | 0.747 |
| Women (M1 n=1035, M2 n=1015) | ||||||||||
| Femoral Neck M1 | 1.03 [−0.87; 2.93] | 0.289 | 0.79 [−0.78; 2.36] | 0.324 | 0.16 [0.04; 0.28] | 0.010 | 0.30 [−1.38; 1.97] | 0.727 | 0.20 [−0.05; 0.46] | 0.117 |
| Femoral Neck M2 | 1.08 [−0.89; 3.05] | 0.283 | 0.47 [−1.15; 2.09] | 0.569 | 0.16 [0.03; 0.29] | 0.013 | 0.49 [−1.24; 2.23] | 0.577 | 0.18 [−0.08; 0.44] | 0.176 |
| Femoral trochanter M1 | 2.32 [0.50; 4.14] | 0.012 | 1.71 [0.20; 3.21] | 0.026 | 0.07 [−0.05; 0.19] | 0.254 | −0.34 [−1.95; 1.26] | 0.674 | 0.25 [0.004; 0.49] | 0.046 |
| Femoral trochanter M2 | 2.72 [0.8; 4.64] | 0.006 | 1.53 [−0.05; 3.11] | 0.058 | 0.08 [−0.04; 0.21] | 0.182 | −0.17 [−1.87; 1.52] | 0.841 | 0.22 [−0.04; 0.48] | 0.094 |
M1: model 1 adjusted for age, education, and time interval between exam and NP assessment.
M2: model 2 adjusted for age, education, time interval between exam and NP assessment, systolic blood pressure, antihypertensive medication use, smoking, diabetes, prevalent CVD, atrial fibrillation, total cholesterol, and triglycerides.
Bold values indicate statistical significance (P<0.05).
β corresponds to the change in cognitive test outcome associated with a 1 unit-increase in BMD.
CI indicates confidence interval; CVD, cardiovascular disease; HVOT, Hooper Visual Organization test; NP, neuropsychological.
Further analysis adjusting for PAI, osteoporosis and antidepressant medications did not greatly change effect sizes, even though significance was lost in certain cases. Adjustment for BMI in women led to a loss of association between trochanter BMD and logical memory, indicating that BMI is a potential confounder (Supplemental Table C, Supplemental Digital Content 2, http://links.lww.com/WAD/A336). When we stratified by high/low BMI category (cut off 30 kg/cm2), we saw no difference in the specific association between those with high versus low BMI (data not shown).
Effect Modification by APOE4 Status
BMD was associated with better Trails B-A performance among women with positive APOE4 status compared with APOE4 negative participants, showing potential protective effect of lifetime estrogen exposure on executive function and processing speed among women with increased risk for neurodegeneration. In men, BMD was linked to better Trails B-A performance irrespective of APOE status (Table 4). Conversely, APOE4 status had no effect on the association between BMD and structural brain changes (data not shown).
TABLE 4.
Stratified Analysis by APOE4 Status in the Association Between BMD Measures and NP Test Performance (Where the Interaction was Significant)
| Trail Making B-A (Log) | HVOT (Log) | |||||||
|---|---|---|---|---|---|---|---|---|
| APOE4 | No APOE4 | APOE4 | No APOE4 | |||||
| BMD Measure | β [95% CI] | P | β [95% CI] | P | β [95% CI] | P | β [95% CI] | P |
| Men (n=847) | ||||||||
| Femoral neck | 0.20 [0.01; 0.39] | 0.043 | 0.15 [0.02; 0.28] | 0.020 | −0.36 [−0.85; 0.13] | 0.150 | 0.14 [−0.13; 0.42] | 0.308 |
| Women (n=1005) | ||||||||
| Femoral neck | 0.40 [0.09; 0.71] | 0.012 | 0.10 [−0.03; 0.24] | 0.144 | 0.32 [−0.25; 0.89] | 0.271 | 0.18 [−0.11; 0.47] | 0.221 |
Adjusted for age, education, and time interval between exam and NP assessment.
Bold values indicate statistical significance (P<0.05).
β corresponds to the change in cognitive test outcome associated with a 1 unit-increase in BMD.
BMD indicates bone mineral density; CI, confidence interval; HVOT, Hooper Visual Organization test; NP, neuropsychological.
DISCUSSION
Our study addresses the association between BMD and structural and cognitive markers of brain aging among nondemented Gen 2 participants of the FHS. Our results show sex-dependent associations, some of them new, that are mostly in accordance with the accepted theory that BMD is linked to cerebral function and structure, most likely, through its role as a biomarker of cumulative estrogen exposure.
Estrogen is a plausible common denominator protecting both bone and brain health; it inhibits bone resorption through receptors found on bone tissue;25 serum estrogen is related to BMD in both premenopausal and postmenopausal women and the MrOs study underscored its role in bone health even among men.26,27 At the same time, estrogen reduces neuronal apoptosis and atherosclerosis, inhibits amyloid-b formation and tau hyperphosphorylation and reduces oxidative stress in the brain.9,28 Despite these neuroprotective observations, administration of estrogen in postmenopausal women has not demonstrated neuroprotective effects29 and does not decrease the risk for incident AD.30 Instead, it has been associated with increased WMHV in the absence of significant cardiovascular risk factors,31 gray matter volume loss,32 lower HVs.33 The negative effects of exogenous estrogen suggest that rather than estrogen alone, factors such as the hormonal and/or neurotrophic milieu, age at exposure and genetic predisposition, may all be important in driving the neuroprotective effects of intrinsic estrogen.
In our study of the “young old” participants (Gen 2, mean age 66) high BMD is linked to less WMHV and better performance on the Trails B-A test, suggesting less cumulative vascular injury to the brain. This association is seen in both sexes and persists even after adjusting for the presence of known cerebrovascular risk factors studied at FHS. Similar observations with WMHV have been described among older individuals (mean age 78) in the Cardiovascular Health Study34 which included both Caucasians and African Americans, as well as in an Asian, community-based cohort of nondemented and stroke-free individuals aged 50 to 75.35 These findings suggest that low BMD may be a surrogate marker of increased risk of cerebrovascular disease even in the absence of known vascular risk factors . The lack of an association between BMD and HV in our study is likely because of our sample of nondemented participants; in a prior study by Luskotova et al36 low BMD was linked to hippocampal atrophy only among participants with early AD, and not among nondemented controls.
We show novel, sex-specific associations between BMD and certain cognitive domains; in particular, women with higher BMD performed better on a verbal memory test and men with higher BMD performed better on a visual memory test. A positive link between femoral BMD and verbal memory has been shown independently in elderly men37 and postmenopausal women.38 In studies that, similar to ours, included both sexes28,39 a sex-specific association was not seen. The link between BMD and visual memory is novel. In cohorts focusing on healthy older individuals, a baseline advantage in verbal memory tasks has been reported in women compared with men40–44 and it has been argued that estrogen may have domain-specific cognitive effects in nondemented women.45 In regards to visuospatial performance, some studies show a male over female advantage42,43 while others show no sex-specific differences.44 Its potential link to estrogen warrants further studies. Understanding the factors underlying verbal and visuospatial memory impairments is very important as they are potential markers of neurodegeneration and may predict the development of dementia and AD.28,46,47 Our results suggest that cumulative estrogen exposure may be protective against vascular damage, regardless of the presence of vascular risk factors. With regards to neuronal degeneration, its effect may differ between the sexes and specific cognitive domains.
We investigated the effect of APOE4 status on the association between BMD and cognition and observed that BMD is linked to better Trails B-A performance in men regardless of their status, but among women the positive association is seen only among those with APOE4 positive. This finding may indicate a protective role for cumulative estrogen exposure against vascular degeneration among women with a genetic predisposition to neurodegeneration.48 It also underscores a probable underestimation of the role of estrogen on cognition among men. Of note, previous studies have not shown a significant effect of APOE4 status on the association between BMD and cognition.39
Discrepancies in the literature compared with our results may be attributable to differences in the studies’ participant age groups, race,4 BMD measurements sites (hip, spine, whole body, etc.), and cognitive performance tests, some of which are considered more crude measures of cognitive function (mini-mental state examination).13,38,49,50 In our study, the majority of associations are noted when BMD is measured at the FN and not at the level of the trochanter with the exception of verbal memory in women where the link is seen when BMD is measured at the trochanter, but not at the FN. The etiology for this discrepancy is unclear. Studies focusing on osteoporosis pattern have shown bone mass loss at both sites with increasing age which is maximal in the early post-enopausal phase.51 However, it has been reported that between sites of BMD measurement the rate of bone loss may vary, and the correlation decreases with increasing age7,52 and with degree of bone loss. Higher between-site correlations were seen in individuals with normal BMD and those with osteopenia compared with those with osteoporosis.53 Another limitation of our study is the primarily Caucasian population of the FHS which limits applicability of the results to other populations. In addition, participants who were included in the analysis, when compared with participants who had BMD, but did not present for NP testing, represent a healthier subgroup with lower prevalence of vascular risk factors and CVD (Supplemental Table A, Supplemental Digital Content 3, http://links.lww.com/WAD/A337). Healthier individuals are more likely to come for multiple ancillary studies in cohort studies.34 Finally, diet which is known to affect both BMD and brain health volumes54 was not considered in our analysis.
Overall, our study expands on previously described associations between BMD and WMH burden in the “young old” independent of known cerebrovascular risk factors. Screening for low BMD in healthy subjects may offer an opportunity for an intervention to decrease the risk of cerebral vascular injury. Our study provides further evidence that the link between BMD and cognition may differ between the sexes and for different cognitive domains, and that this link may be further modulated by the presence of a genetic predisposition towards neurodegeneration. Further studies to address molecular interactions on how estrogen affects bone and brain health may help create more effective interventions for those at higher risk for developing neurodegenerative diseases.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.alzheimerjournal.com.
Footnotes
This study was feasible through grant support from NIH R01AG054076-02.
M.S. drafted the manuscript. Concept design, data acquisition, analysis and interpretation of findings was performed by M.S., A.S.B., J.J.H., S.S. and critical revision by A.S.B., J.J.H., C.D.C., C.S., and S.S. Statistical analysis was performed by A.O.D., J.J.H., and A.S.B.
The authors declare no conflicts of interest.
Contributor Information
Maria Stefanidou, Email: mastefan@bu.edu.
Jayandra J. Himali, Email: jhimali@bu.edu.
Charles DeCarli, Email: cdecarli@ucdavis.edu.
Claudia Satizabal, Email: satizabal@uthscsa.edu.
Alexa S. Beiser, Email: alexab@bu.edu.
Sudha Seshadri, Email: seshadri@uthscsa.edu.
REFERENCES
- 1. Sato Y. Dementia and fracture. Clin Calcium. 2010;20:1379–1384. [PubMed] [Google Scholar]
- 2. Liu D, Zhou H, Tao Y, et al. Alzheimer’s disease is associated with increased risk of osteoporosis: the Chongqing Aging Study. Curr Alzheimer Res. 2016;13:1165–1172. [DOI] [PubMed] [Google Scholar]
- 3. Lee DY, Na DL, Seo SW, et al. Association between cognitive impairment and bone mineral density in postmenopausal women. Menopause. 2012;19:636–641. [DOI] [PubMed] [Google Scholar]
- 4. Bae IS, Kim JM, Cheong JH, et al. Association between bone mineral density and brain parenchymal atrophy and ventricular enlargement in healthy individuals. Aging (Albany NY). 2019;11:8217–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Basgoz B, Ince S, Safer U, et al. Low bone density and osteoporosis among older adults with Alzheimer’s disease, vascular dementia, and mixed dementia: a cross-sectional study with prospective enrollment. Turk J Phys Med Rehabil. 2020;66:193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanyu H, Sugiyama T, Abe S, et al. Abnormality of bone mineral metabolism in elderly female patients with dementia. Nihon Ronen Igakkai Zasshi. 1993;30:857–863. [DOI] [PubMed] [Google Scholar]
- 7. Tan ZS, Seshadri S, Beiser A, et al. Bone mineral density and the risk of Alzheimer disease. Arch Neurol. 2005;62:107–111. [DOI] [PubMed] [Google Scholar]
- 8. Zhou R, Zhou H, Rui L, et al. Bone loss and osteoporosis are associated with conversion from mild cognitive impairment to Alzheimer’s disease. Curr Alzheimer Res. 2014;11:706–713. [DOI] [PubMed] [Google Scholar]
- 9. Fox M, Berzuini C, Knapp LA. Cumulative estrogen exposure, number of menstrual cycles, and Alzheimer’s risk in a cohort of British women. Psychoneuroendocrinology. 2013;38:2973–2982. [DOI] [PubMed] [Google Scholar]
- 10. Baldini V, Mastropasqua M, Francucci CM, et al. Cardiovascular disease and osteoporosis. J Endocrinol Invest. 2005;28(suppl):69–72. [PubMed] [Google Scholar]
- 11. NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–795.11176917 [Google Scholar]
- 12. Tan ZS, Spartano NL, Beiser AS, et al. Physical activity, brain volume, and dementia risk: the Framingham study. J Gerontol A Biol Sci Med Sci. 2017;72:789–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Laudisio A, Fontana DO, Rivera C, et al. Bone mineral density and cognitive decline in elderly women: results from the InCHIANTI study. Calcif Tissue Int. 2016;98:479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Au R, Seshadri S, Wolf PA, et al. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp Aging Res. 2004;30:333–358. [DOI] [PubMed] [Google Scholar]
- 15. Farmer ME, White LR, Kittner SJ, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60(pt 2):1023–1040. [DOI] [PubMed] [Google Scholar]
- 16. Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35:1857–1861. [DOI] [PubMed] [Google Scholar]
- 17. Seshadri S, Wolf PA, Beiser AS, et al. Association of plasma total homocysteine levels with subclinical brain injury: cerebral volumes, white matter hyperintensity, and silent brain infarcts at volumetric magnetic resonance imaging in the Framingham Offspring Study. Arch Neurol. 2008;65:642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fletcher E, Singh B, Harvey D, et al. Adaptive image segmentation for robust measurement of longitudinal brain tissue change. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:5319–5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. DeCarli C, Fletcher E, Ramey V, et al. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fletcher E, Carmichael O, Decarli C. MRI non-uniformity correction through interleaved bias estimation and B-spline deformation with a template. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:106–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the framingham heart study: establishing what is normal. Neurobiol Aging. 2005;26:491–510. [DOI] [PubMed] [Google Scholar]
- 22. Segev D, Hellerstein D, Dunsky A. Physical activity-does it really increase bone density in postmenopausal women? A review of articles published between 2001-2016. Curr Aging Sci. 2018;11:4–9. [DOI] [PubMed] [Google Scholar]
- 23. De Laet C, Kanis JA, Oden A, et al. Body mass index as a predictor of fracture risk: a meta-analysis. Osteoporos Int. 2005;16:1330–1338. [DOI] [PubMed] [Google Scholar]
- 24. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29:1243–1251. [DOI] [PubMed] [Google Scholar]
- 25. Loskutova N, Honea RA, Vidoni ED, et al. Bone density and brain atrophy in early Alzheimer’s disease. J Alzheimers Dis. 2009;18:777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barrett-Connor E, Laughlin GA, Li H, et al. The association of concurrent vitamin D and sex hormone deficiency with bone loss and fracture risk in older men: the osteoporotic fractures in men (MrOS) study. J Bone Miner Res. 2012;27:2306–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LeBlanc ES, Nielson CM, Marshall LM, et al. The effects of serum testosterone, estradiol, and sex hormone binding globulin levels on fracture risk in older men. J Clin Endocrinol Metab. 2009;94:3337–3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Y, Seshadri S, Ellison RC, et al. Bone mineral density and verbal memory impairment: Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2001;154:795–802. [DOI] [PubMed] [Google Scholar]
- 29. McCarrey AC, Resnick SM. Postmenopausal hormone therapy and cognition. Horm Behav. 2015;74:167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Seshadri S, Zornberg GL, Derby LE, et al. Postmenopausal estrogen replacement therapy and the risk of Alzheimer disease. Arch Neurol. 2001;58:435–440. [DOI] [PubMed] [Google Scholar]
- 31. Kantarci K, Tosakulwong N, Lesnick TG, et al. Brain structure and cognition 3 years after the end of an early menopausal hormone therapy trial. Neurology. 2018;90:e1404–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang T, Casanova R, Resnick SM, et al. Effects of hormone therapy on brain volumes changes of postmenopausal women revealed by optimally-discriminative voxel-based morphometry. PLoS One. 2016;11:e0150834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maki PM, Henderson VW. Hormone therapy, dementia, and cognition: the Women’s Health Initiative 10 years on. Climacteric. 2012;15:256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barzilay JI, Buzkova P, Fink HA, et al. Systemic markers of microvascular disease and bone mineral density in older adults: the cardiovascular health study. Osteoporos Int. 2016;27:3217–3225. [DOI] [PubMed] [Google Scholar]
- 35. Minn YK, Suk SH, Do SY. Osteoporosis as an independent risk factor for silent brain infarction and white matter changes in men and women: the PRESENT project. Osteoporos Int. 2014;25:2465–2469. [DOI] [PubMed] [Google Scholar]
- 36. Loskutova N, Honea RA, Brooks WM, et al. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer’s disease. J Alzheimers Dis. 2010;20:313–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Catalano A, Sardella A, Bellone F, et al. Executive functions predict fracture risk in postmenopausal women assessed for osteoporosis. Aging Clin Exp Res. 2019;32:2251–2257. [DOI] [PubMed] [Google Scholar]
- 38. Brownbill RA, Ilich JZ. Cognitive function in relation with bone mass and nutrition: cross-sectional association in postmenopausal women. BMC Womens Health. 2004;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sohrabi HR, Bates KA, Weinborn M, et al. Bone mineral density, adiposity, and cognitive functions. Front Aging Neurosci. 2015;7:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Hooren SA, Valentijn AM, Bosma H, et al. Cognitive functioning in healthy older adults aged 64-81: a cohort study into the effects of age, sex, and education. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:40–54. [DOI] [PubMed] [Google Scholar]
- 41. Chapman RM, Mapstone M, Gardner MN, et al. Women have farther to fall: gender differences between normal elderly and Alzheimer’s disease in verbal memory engender better detection of Alzheimer’s disease in women. J Int Neuropsychol Soc. 2011;17:654–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wiederholt WC, Cahn D, Butters NM, et al. Effects of age, gender and education on selected neuropsychological tests in an elderly community cohort. J Am Geriatr Soc. 1993;41:639–647. [DOI] [PubMed] [Google Scholar]
- 43. de Frias CM, Nilsson LG, Herlitz A. Sex differences in cognition are stable over a 10-year period in adulthood and old age. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2006;13:574–587. [DOI] [PubMed] [Google Scholar]
- 44. Brunet HE, Caldwell JZK, Brandt J, et al. Influence of sex differences in interpreting learning and memory within a clinical sample of older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2020;27:18–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Buckwalter JG, Rizzo AA, McCleary R, et al. Gender comparisons of cognitive performances among vascular dementia, Alzheimer disease, and older adults without dementia. Arch Neurol. 1996;53:436–439. [DOI] [PubMed] [Google Scholar]
- 46. Iachini I, Iavarone A, Senese VP, et al. Visuospatial memory in healthy elderly, AD and MCI: a review. Curr Aging Sci. 2009;2:43–59. [DOI] [PubMed] [Google Scholar]
- 47. Kuh D, Cooper R, Moore A, et al. Age at menopause and lifetime cognition: findings from a British birth cohort study. Neurology. 2018;90:e1673–e1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Laws KR, Irvine K, Gale TM. Sex differences in cognitive impairment in Alzheimer’s disease. World J Psychiatry. 2016;6:54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kang HG, Park HY, Ryu HU, et al. Bone mineral loss and cognitive impairment: the PRESENT project. Medicine (Baltimore). 2018;97:e12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel A, Jameson KA, Edwards MH, et al. Mild cognitive impairment is associated with poor physical function but not bone structure or density in late adulthood: findings from the Hertfordshire cohort study. Arch Osteoporos. 2018;13:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hedlund LR, Gallagher JC. The effect of age and menopause on bone mineral density of the proximal femur. J Bone Miner Res. 1989;4:639–642. [DOI] [PubMed] [Google Scholar]
- 52. Namwongprom S, Ekmahachai M, Vilasdechanon N, et al. Bone mineral density: correlation between the lumbar spine, proximal femur and Radius in northern Thai women. J Med Assoc Thai. 2011;94:725–731. [PubMed] [Google Scholar]
- 53. Choe HS, Lee JH, Min DK, et al. Comparison of vertebral and femoral bone mineral density in adult females. J Phys Ther Sci. 2016;28:1928–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Croll PH, Voortman T, Ikram MA, et al. Better diet quality relates to larger brain tissue volumes: the Rotterdam study. Neurology. 2018;90:e2166–e2173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.alzheimerjournal.com.


