FIG 5.
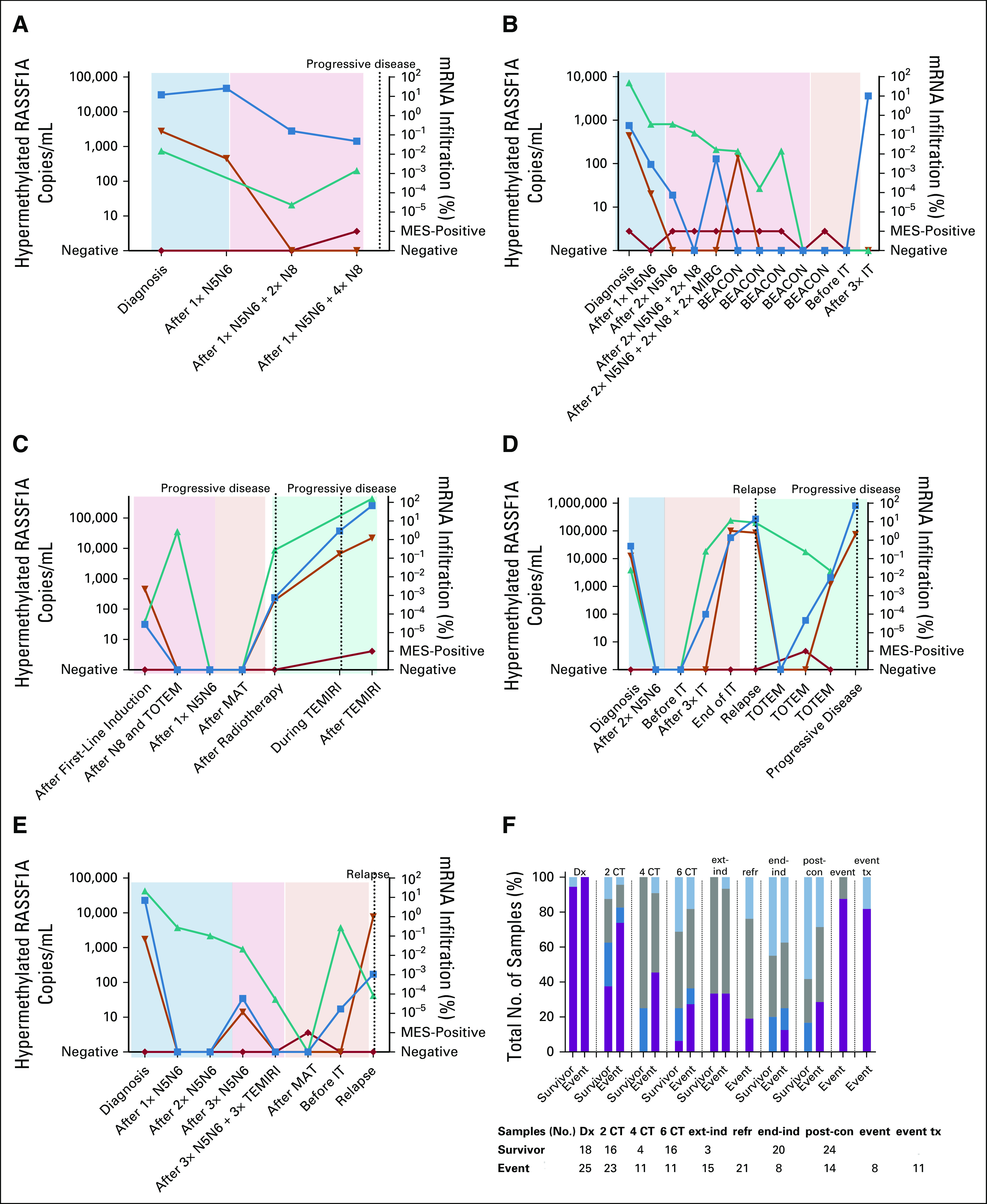
(A-E) For patients with refractory, relapse, or progressive disease, all sequential samples, if available, were analyzed for hypermethylated RASSF1A (blue squares; N2063, N2071, N2099, N2101, and N2123, respectively). Corresponding blood (orange triangles) and BM (green triangles for adrenergic markers and red diamonds for MES markers) samples were tested for mRNA. Colored blocks indicate the treatment: light blue, induction therapy; light red, extra induction therapy; light orange, postconsolidation therapy; light green, relapse or progressive disease treatment. (F) Fraction of total number of tested samples, which were positive for circulating hypermethylated RASSF1A and/or BM mRNA, of patients who will suffer an event compared with those who remain in complete remission (survivor). Purple bar represents hypermethylated RASSF1A+ and mRNA panel+ samples, dark blue bar represents hypermethylated RASSF1A ctDNA+ and mRNA panel– samples, gray bar represents hypermethylated RASSF1A– and mRNA panel+ samples, and light blue bar represents hypermethylated RASSF1A ctDNA–/mRNA panel– samples. BEACON, TEMIRI, and TOTEM are treatment for refractory or relapsed disease. BEACON, BEACON-Neuroblastoma Trial: bevacizumab, temozolomide ± irinotecan; BM, bone marrow; CT, cycles of chemotherapy; ctDNA; circulating tumor DNA; Dx, diagnosis; end-ind, end of induction; event tx, event therapy; ext-ind, extra induction therapy (not for refractory disease); IT, immunotherapy; MAT, myeloablative therapy; MES, mesenchymal; MIBG, iodine-131-meta-iodobenzylguanidine; N5, N6, and N8; courses of induction chemotherapy; post-con, postconsolidation; refr, refractory disease treatment; TEMIRI, temozolomide and irinotecan; TOTEM, temozolomide and topotecan.
