Acute cellular rejection (ACR) after heart transplantation (HTx) is a T cell-mediated process diagnosed histologically by endomyocardial biopsy (EMB) with severity ranging from 0R (no rejection), 1R (mild rejection), 2R (moderate rejection), to 3R (severe rejection) with symptomatic 1R ACR and any ACR ≥ 2R warranting treatment with corticosteroids.1 Beyond basic characterization of immune infiltrates on histology, the specific mechanisms behind ACR remain poorly understood. Programmed death ligand 1 (PD-L1) on host cells interacts with programmed cell death protein 1 (PD-1) on immune cells to inhibit T cell activity.2 Recent data from patients with malignancy who developed myocarditis following treatment with anti-PD-1 therapies demonstrate robust upregulation of PD-L1 in cardiomyocytes, underscoring a critical role for PD-1/PD-L1 signaling in cardiovascular-immune tolerance.3 We hypothesize that PD-L1 is differentially upregulated in cardiac tissue in the setting of ACR.
Nineteen patients with EMB samples corresponding to 0R, 1R, and 2R ACR were analyzed for percentage of cardiomyocytes expressing PD-L1 using previously validated immunohistochemistry and image analysis techniques.4 Representative images from this analysis are shown in Figure 1A. Demographic information was collected on these patients as well as right heart catherization (RHC), electrocardiography (EKG), transthoracic echocardiogram (TTE), and laboratory data within 3 days of EMB. The study was approved by Vanderbilt’s Institutional Review Board and each patient gave informed consent prior to cardiac biopsy. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Figure 1.
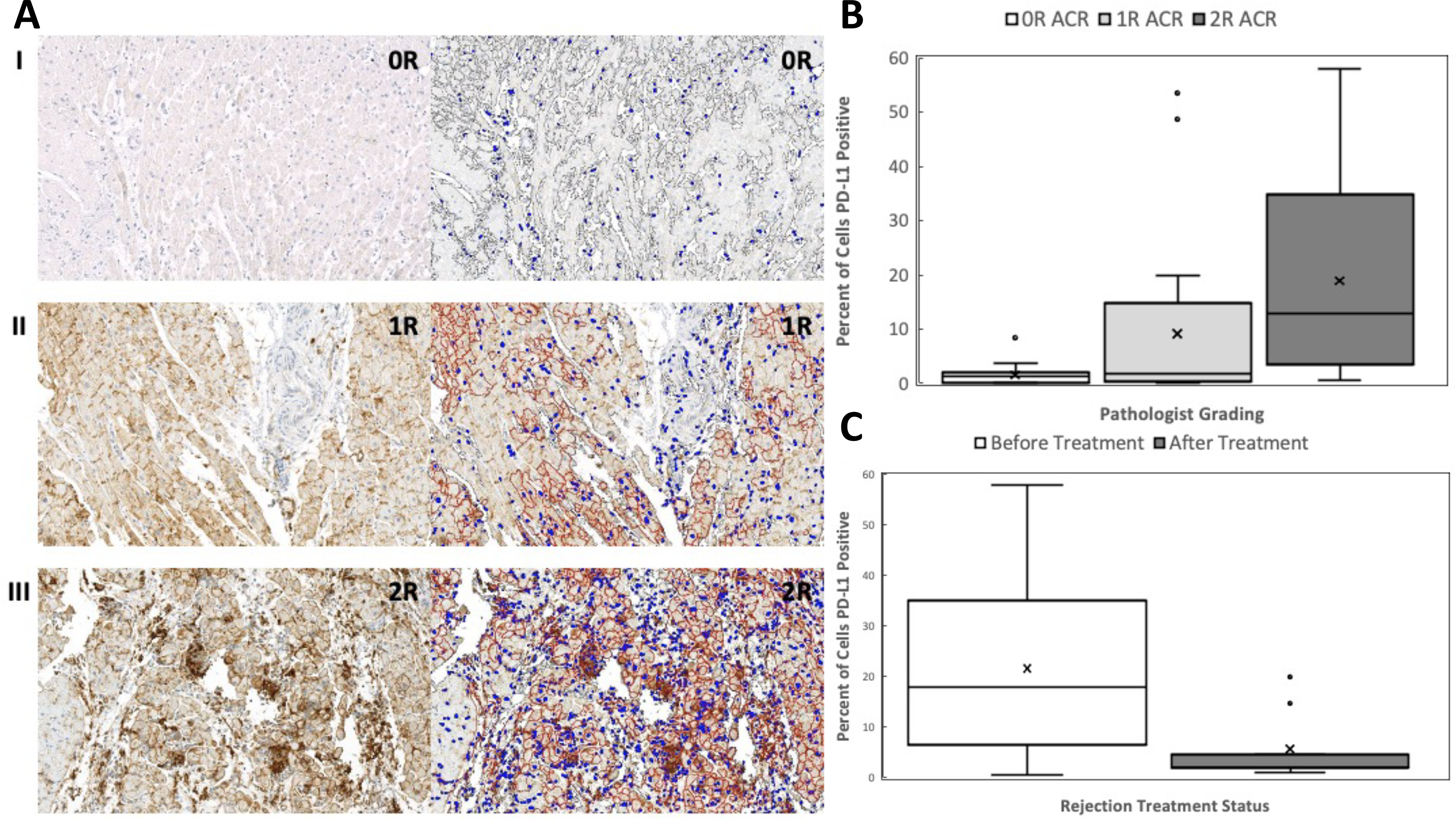
Percent of cells expressing PD-L1. (A) Representative images of PD-L1 expression for patient 5. Images on left represent original staining after PD-L1 immunohistochemistry. Images on right represent alterations after manual annotation and software markup looking for PD-L1 positive cells. Brown is considered positive signal for PD-L1 expression. Blue depicts nuclei. (I) 10X magnification, 0R ACR, percent positive cells = 0.1. (II) 10X magnification, 1R ACR, percent positive cells = 15.42. (III) 10X magnification, 2R ACR, percent positive cells = 34.81. (B) Percent of cells expressing PD-L1 significantly differs between 0R and 2R rejection (P < 0.0001) as well as 1R and 2R rejection (P = 0.015) but not 0R and 1R rejection (P = 0.091). (C) Percent of cell expressing PD-L1 significantly decreases after treatment of ACR (paired Wilcoxon, P = 0.039).
In our cohort of nineteen patients, the majority of donors were male (68.4%) with a median age of 29 [23–37] years. The recipients had a median age of 48 [45–58] years with most being male (73.7%) and white (84.2%). The most common indication for transplantation was non-ischemic cardiomyopathy (63.2%); common comorbidities included hypertension (94.7%), hyperlipidemia (94.7%), chronic kidney disease (84.2%), diabetes (47.4%), and smoking history (47.4%). In addition to rejection, based on chart review up to December 2019, other complications included cardiac allograft vasculopathy ≥ 1 based on the ISHLT grading system (36.8%), infection requiring hospitalization (21.1%), development of malignancy (10.5%), and death (10.5%).
Median percentage of cardiomyocytes expressing PD-L1 for 0R, 1R, and 2R ACR was 1.3 [0.11–2.0], 1.8 [0.32–14.7], and 12.9 [3.6–34.8], respectively (Kruskal-Wallis p<0.0001) (Figure 1B). Post-hoc pairwise analysis found significant differences in PD-L1 expressing cells between 0R vs. 2R rejection (P < 0.0001) and 1R vs. 2R rejection (P = 0.015) but not 0R vs. 1R rejection (P = 0.091). Nine patients who were treated for 2R ACR with increased immunosuppression had post-treatment EMB samples available for analysis. In these patients, the percentage of PD-L1 expressing cells decreased significantly from 18.0 [6.5–35.2] before treatment to 2.1 [2.0–4.7] after treatment (paired Wilcoxon p=0.039) (Figure 1C).
To see if PD-L1 expression from each EMB correlated with other clinical endpoints, we assessed several biomarkers taken within 3 days of that EMB including invasive hemodynamics from RHC, EKG findings, TTE data, and common laboratory values. An ordinal regression accounting for age and sex demonstrated that PD-L1 expression was significantly associated with mean pulmonary artery (PA) pressure (OR 2.4 [1.4–4.1]), pulmonary capillary wedge (PCW) pressure (OR 2.0 [1.4–2.9]), and B-type natriuretic peptide (BNP) (OR 1.6 [1.2–2.1]).
Our study reveals that PD-L1 expression is upregulated across cardiomyocytes in proportion to the severity of ACR after transplantation. Successful treatment of ACR with increased immunosuppression subsequently decreases PD-L1 expression. Moreover, PD-L1 expression correlates with hemodynamics (increased PA and PCW pressures) and established biomarkers (BNP) of myocardial dysfunction, supporting PD-L1 expression as a potentially clinically meaningful biomarker for detecting both cellular rejection and improvement after treatment. These findings support the role of PD-L1/PD-1 interaction as an important modulator of how the immune system interacts with the heart.
The main limitation of this study was its single-center, observational nature. We used paired samples to mitigate variation given the relatively small number of cases. However, it is possible that foci of lymphocytes were adjacent to biopsy samples and thus not apparent in staining. This could have influenced initial grading of ACR by pathologists as well as PD-L1 expression. Furthermore, we cannot presume that PD-L1 upregulation is a specific response to ACR as opposed to other forms of injury. Finally, we cannot exclude the effects of harvest injury on samples taken within the first few weeks of transplantation as well as the impact of differing rejection sequence on PD-L1 expression.
Though the results from this study do not obviate need for surveillance EMB in detecting ACR, one future avenue for our work could involve tracking soluble PD-L1 levels in the serum of patients during ACR episodes to see if increased myocardial PD-L1 expression translates to increased serum soluble PD-L1 levels.5 The results of such a study could potentially provide an alternative option to biopsy for detecting ACR after HTx.
Acknowledgments
Sources of Funding:
JM and this work was supported by National Institutes of Health grants (R01HL141466, R01HL155990, and R01HL156021).
Disclosure statements:
JES has served as a consultant for Bristol-Myers Squibb. JL has served as a consultant for Abbott, AstraZeneca, CVRx, Boehringer Ingelheim, Edwards LifeSciences, Impulse Dynamics, and VWave; and is supported by grants from Astra Zeneca, Volumetric, Sensible Medical. JM has served on advisory boards for Bristol Myers Squibb, Takeda, Regeneron, Audentes, Deciphera, Ipsen, Janssen, Immuno-Core, Boston Biomedical, Amgen, Myovant, Triple Gene/Precigen, Cytokinetics, and AstraZeneca. All other authors have reported that they have no relationships to disclose relevant to the contents of this paper.
Non-standard Abbreviations and Acronyms:
- ACR
Acute cellular rejection
- BNP
B-type natriuretic peptide
- EKG
Electrocardiography
- EMB
Endomyocardial biopsy
- HTx
Heart transplantation
- PA
Pulmonary artery
- PCW
Pulmonary capillary wedge
- PD-1
Programmed cell death protein
- PD-L1
Programmed death ligand 1
- RHC
Right heart catherization
- TTE
Transthoracic echocardiogram
References:
- 1.Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, Demetris AJ, Hammond E, Itescu S, Marboe CC, McManus B, Reed EF, Reinsmoen NL, Rodriguez ER, Rose AG, Rose M, Suciu-Focia N, Zeevi A and Billingham ME. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–20. [DOI] [PubMed] [Google Scholar]
- 2.Moslehi J, Lichtman AH, Sharpe AH, Galluzzi L and Kitsis RN. Immune checkpoint inhibitor-associated myocarditis: manifestations and mechanisms. J Clin Invest. 2021;131: e145186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA Jr., Anders RA, Sosman JA and Moslehi JJ. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med. 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sunshine JC, Nguyen PL, Kaunitz GJ, Cottrell TR, Berry S, Esandrio J, Xu H, Ogurtsova A, Bleich KB, Cornish TC, Lipson EJ, Anders RA and Taube JM. PD-L1 Expression in Melanoma: A Quantitative Immunohistochemical Antibody Comparison. Clin Cancer Res. 2017;23:4938–4944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ugurel S, Schadendorf D, Horny K, Sucker A, Schramm S, Utikal J, Pfohler C, Herbst R, Schilling B, Blank C, Becker JC, Paschen A, Zimmer L, Livingstone E, Horn PA and Rebmann V. Elevated baseline serum PD-1 or PD-L1 predicts poor outcome of PD-1 inhibition therapy in metastatic melanoma. Ann Oncol. 2020;31:144–152. [DOI] [PubMed] [Google Scholar]


