Figure 2: REV7 Controls Pathway Choice at Stalled Replication Forks.
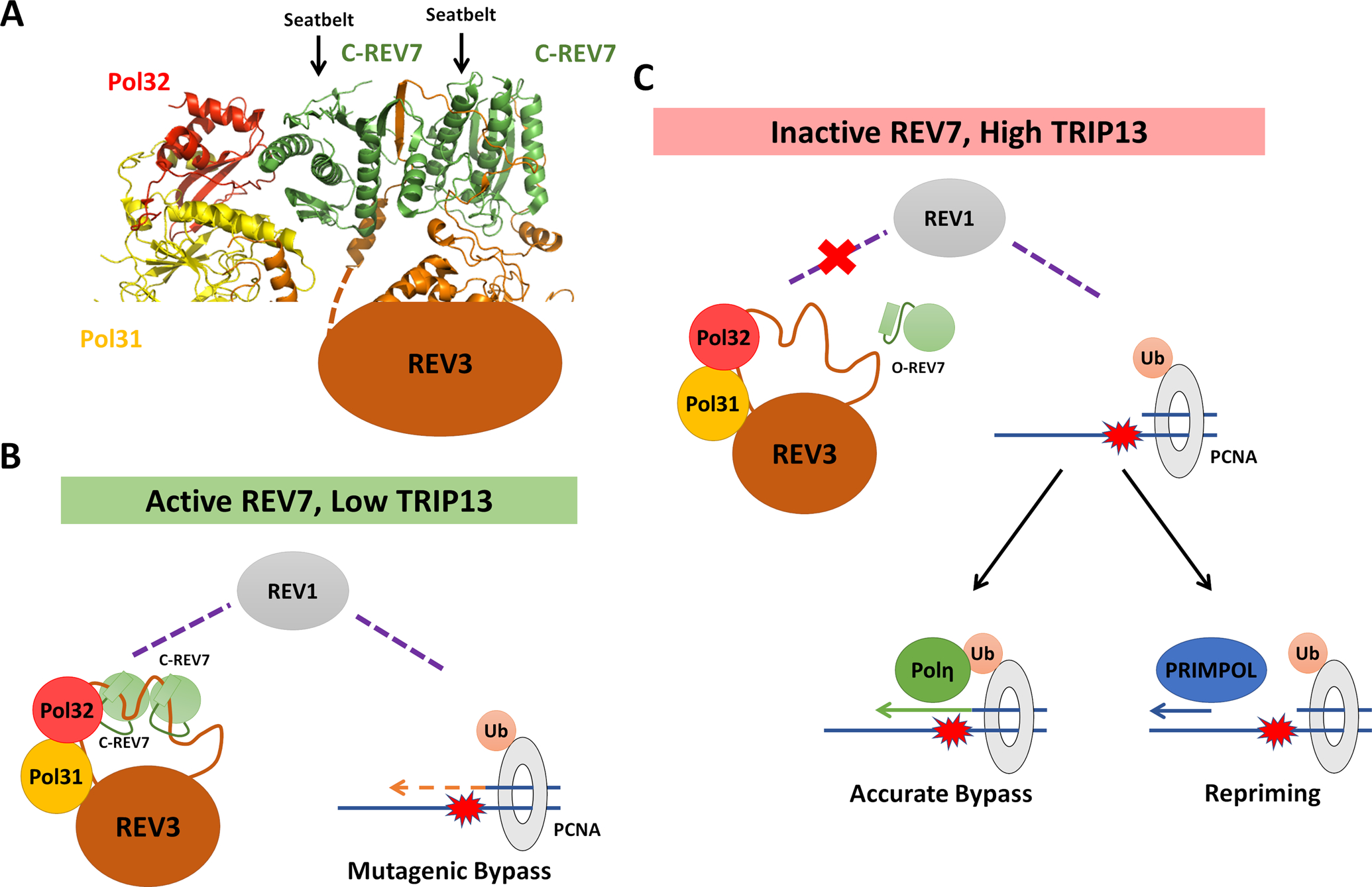
(A) Cryo-electron microscopy structure of the S. cerevisiae DNA Polymerase ζ holoenzyme, consisting of REV3, Pol31, Pol32 and two REV7 molecules (PDB ID: 6V93). REV7 homodimerizes in a head-to-tail manner, forming closed seatbelt-dependent interactions with two seatbelt binding motifs in REV3. One molecule of REV7 makes apparent contract with the Pol32 subunit (POLD3 in mammals). (B) When REV7 is active, whether due to low TRIP13 or other factors, active Polζ is formed with C-REV7 mediating its recruitment to stalled replication forks via REV1. Polζ, together with REV1, catalyzes mutagenic bypass of DNA lesions. (C) When levels of closed REV7 are low, Polζ recruitment is expected to be decreased due to its inability to interact with REV1. In this case, alternative pathways including accurate translesion synthesis and re-priming are expected to predominate in lesion bypass.
