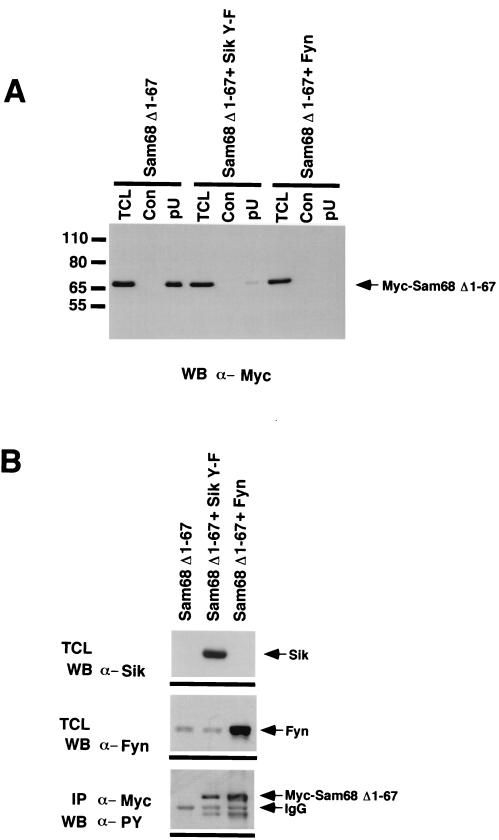
FIG. 7.
Sik negatively regulates the ability of Sam68 to bind RNA. (A) HeLa cell lysates from cells either transfected with Myc-Sam68Δ1–67 alone or cotransfected with Myc-Sam68Δ1–67 and Sik Y-F or Fyn were divided equally and precipitated with agarose (Con) or poly(U)-agarose (pU), followed by anti-Myc immunoblotting. An aliquot of total-cell lysate (TCL) was also included to monitor expression of Myc-Sam68Δ1–67. The ability of Myc-Sam68Δ1–67 to bind poly(U) agarose was inhibited in cells transfected with either Sik Y-F or Fyn. (B) Sik and Fyn are efficiently expressed and phosphorylate Myc-Sam68Δ1–67 in transfected cells. Cell lysates from transfected cells were immunoblotted with anti-Sik and anti-Fyn antibodies to demonstrate that these kinases were efficiently expressed. Immunoprecipitation of Myc-Sam68Δ1–67 followed by immunoblotting with antiphosphotyrosine confirmed that Myc-Sam68Δ1–67 was tyrosine phosphorylated by both Sik and Fyn.