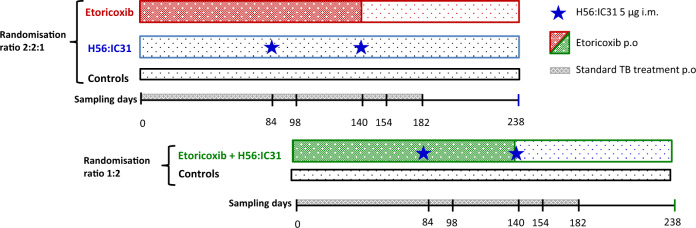
Fig. 5. Study design.
The TBCOX2 study was designed as a randomized, open label, controlled, four group multi-center phase I/II clinical trial with a final allocation ratio 1:1:1:1 to adjunctive interventions. Target number of participants was 10 per arm for the full analysis set (FAS). The first allocation ratio was 2:2:1:0 to (i) etoricoxib, (ii) H56:IC31 and (iii) controls. The second allocation, initiated following a passed interim analysis of safety (when the last participant in the first allocation had reached study day 98), had a ratio of 0:0:1:2 to (iii) controls and (iv) etoricoxib+H56:IC31. Oral administration (p.o.) of etoricoxib 120 mg was initiated within five days of initiation of standard tuberculosis (TB) treatment (182 days), and continued for 140 days. H56:IC31 5 μg intramuscular (i.m) was administered at day 84 and 140. Samples for immunogenicity analyses were harvested at denoted study days.