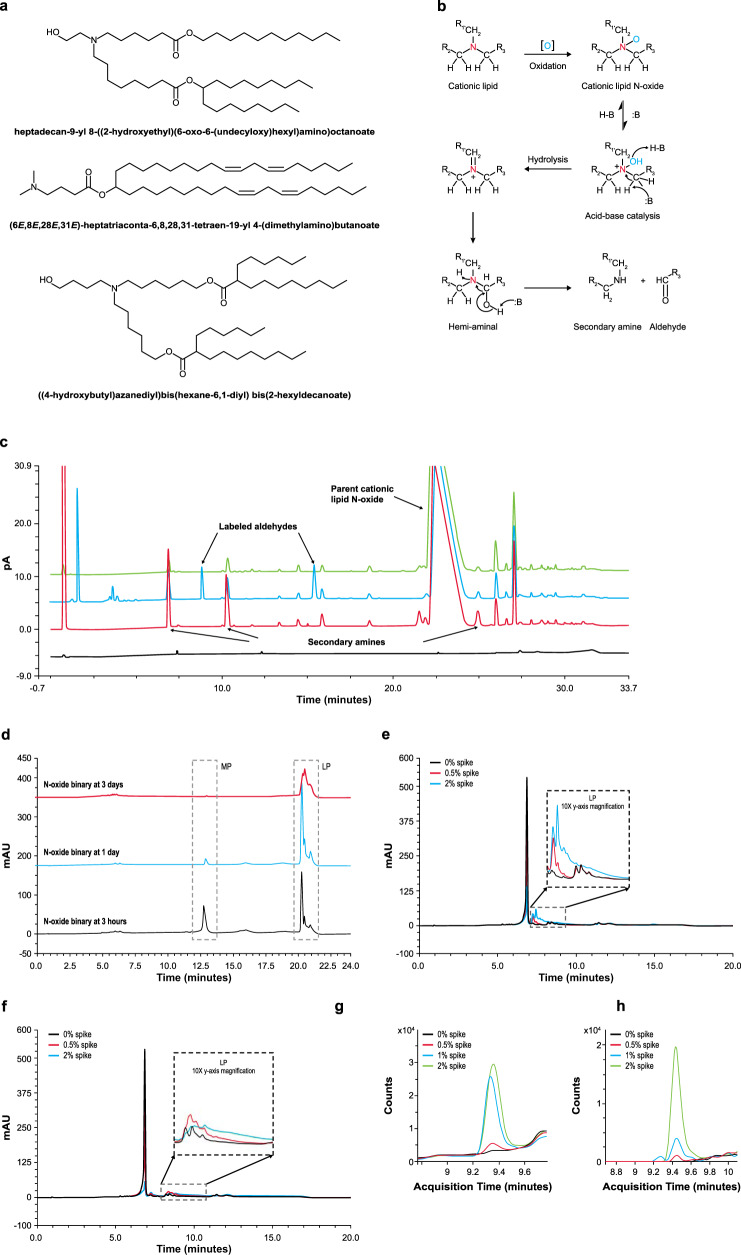
Fig. 5. N-oxide as a driver of adduct formation.
a Tertiary-amine-containing ionizable lipids currently used in RNA-lipid nanoparticle (RNA-LNP) products. b N-oxide forms through tertiary amine oxidation and undergoes an acid/base-catalyzed hydrolysis at the amine to generate aldehydes and secondary amines. c N-oxide acid hydrolysis products were detected by reversed phase-ultra-high-performance liquid chromatography with charged aerosol detection and tandem mass spectrometry (RP-UPLC-CAD MS/MS); N-oxide standard (green), acid-precipitated N-oxide standard with (blue) and without (red) aminooxy-polyethylene glycol (aminooxy-PEG) label, and buffer baseline (black). Secondary amines from N-oxide hydrolysis elute at 8.7, 10.3, and 25 min. Corresponding aminooxy-PEG-derivatized aldehydes elute at 9.5 and 16 min, with the third likely in the column void. pA, pico ampere. d Binary reactions of mRNA with pure N-oxide result in high late eluting-peak (LP). RP-IP analysis of mRNA extracted from the binary is shown at 3 h (black), 1 day (blue), and 3 days (red) MP, mRNA main peak. e Binary reactions spiked with pure 17-carbon aldehyde and f pure 25-carbon aldehyde products from the N-oxide degradation pathway result in high levels of mRNA modification. Reversed phase-ion pair high performance liquid chromatography (RP-IP HPLC) chromatograms of the extracted mRNA are shown with no aldehyde spike (black), 0.5% aldehyde (red), and 2% aldehyde (green). g, h Binary reactions with the pure 17-carbon aldehyde from the N-oxide degradation pathway were analyzed for single nucleoside modifications. Binaries were prepared with no aldehyde (black), 0.5% aldehyde (red), 1% aldehyde (blue), and 2% aldehyde (green) spike. mRNA was extracted, enzyme-digested, and analyzed by liquid chromatography with tandem mass spectrometry (LC-MS/MS). The mass-to-charge ratios (m/z) corresponding to aldehyde-cytidine adducts (m/z 526.3 and 540.3) increased with aldehyde spike level. A representative ionizable lipid system, heptadecan-9-yl 8-((2-hydroxyethyl)(6-oxo-6-(undecyloxy)hexyl)amino)octanoate is used here. Representative data from ≥ 3 repeat experiments are shown.