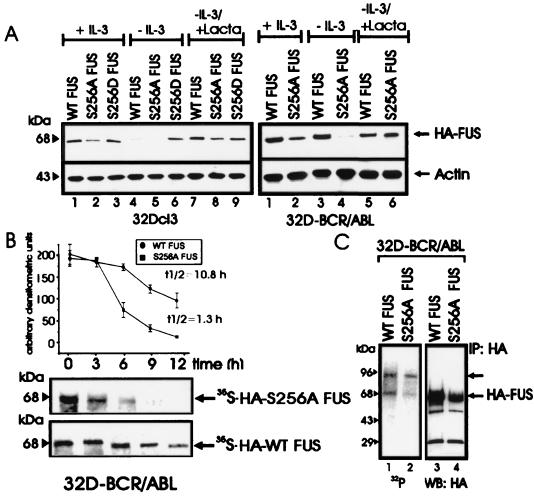
FIG. 4.
Role of serine 256 in expression, stability, proteasome-mediated degradation, and in vivo phosphorylation of FUS. (A) HA-FUS levels in parental (left panel) and BCR-ABL-expressing (right panel) 32Dcl3 cells stably expressing WT FUS or the S256A or S256D mutant. Cells were maintained in the presence of IL-3 or were IL-3 deprived (for 8 h) in the presence or absence of the proteasome inhibitor lactacystin (Lacta). Actin levels were used as a control. (B) Stability of newly synthesized WT FUS and S256A FUS mutant in IL-3- and serum-deprived (8 h) 32DBCR-ABL cells. Each point of the graph represents the mean and standard deviation of the relative amounts of WT FUS and S256A FUS during the chase period. Values on the graph are representatives of three independent experiments. (C) FUS phosphorylation in in vivo 32P-labeled WT FUS- and S256A-expressing 32DBCR-ABL cells (lanes 1 and 2), and amount of immunoprecipitated (IP) WT and S256A FUS (lanes 3 and 4). Data are representative of three experiments with similar results.