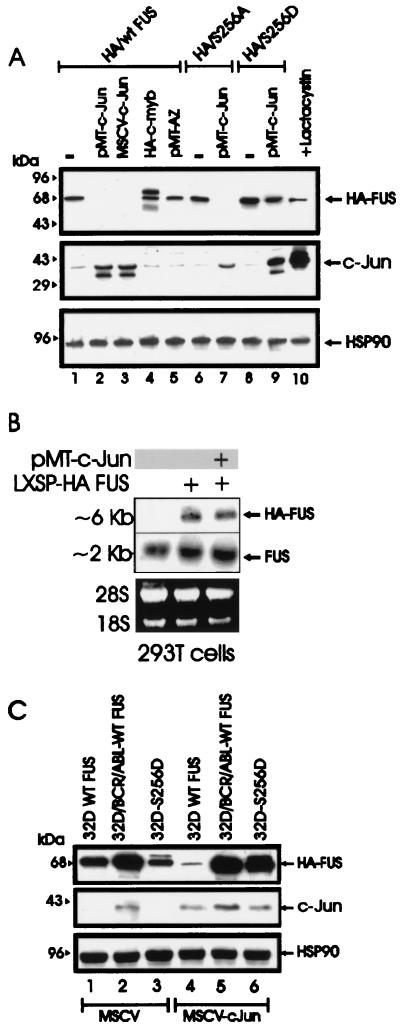
FIG. 5.
c-Jun requirement for FUS proteasome-dependent degradation. (A) HA-FUS expression (top panel) in transiently transfected 293T cells (lane 1), cotransfected with two different c-Jun expression plasmids (pMT-c-Jun [pMT35] and MSCV-c-Jun) (lanes 2 and 3, respectively), with pMT HA-c-Myb (lane 4), or with a CMV-based vector containing the full-length antizyme (AZ) cDNA (lane 5). Expression of S256A FUS and S256D FUS mutants upon transient transfection in 293T cells (lanes 6 and 8) or cotransfection with pMT-c-Jun (lanes 7 and 9) is also shown. 293T cells were also cotransfected with WT HA FUS and pMT-c-Jun and treated for 8 h with the proteasome inhibitor lactacystin before being subjected to lysis (lane 10). c-Jun (middle panel) and HSP90 (bottom panel) expression were monitored as controls. (B) Ectopic (top panel) and endogenous (middle panel) FUS mRNA expression in parental 293T cells (lane 1) or in cells transfected with the LXSP HA-FUS plasmid alone (lane 2) or cotransfected with pMT-c-Jun (lane 3). Ethidium bromide staining of rRNA is shown as a control for equal loading (bottom panel). (C) Effect of transient expression of c-Jun (middle panel) on HA-FUS levels (top panel) in retrovirus-infected parental or BCR-ABL-expressing 32Dcl3 cells constitutively expressing HA-tagged WT FUS or S256D FUS. HSP90 levels (bottom panel) were monitored as a control of equal loading.