Fig. 1.
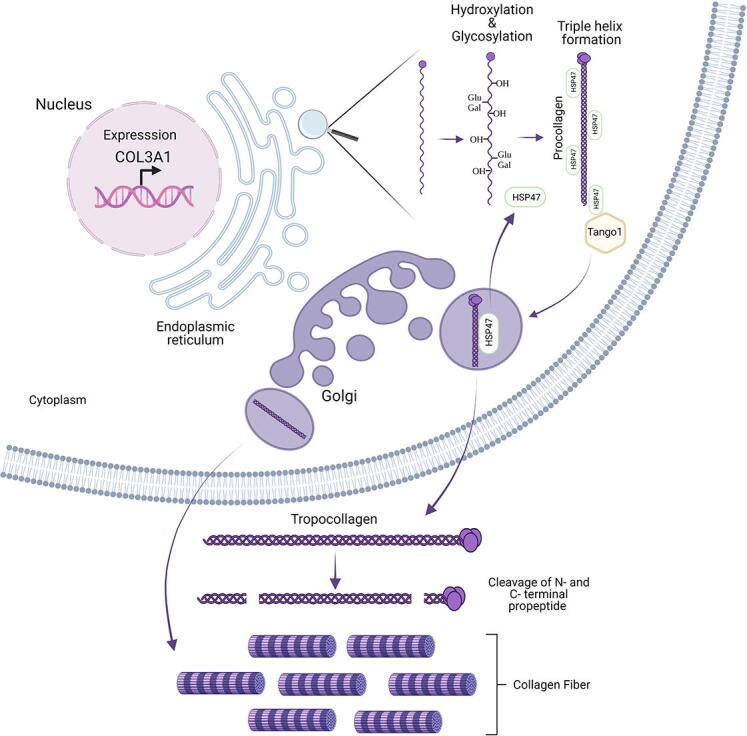
Overview of collagen III protein domain structure and processing by N- and C-proteinases to generate triple helical collagen III that forms heterotypic fibrils with collagen I. Collagen secretion contains several key stages including expression and folding in the ER where the nascent α chains are post-translationally modified by hydroxylation of proline and lysine residues, as well as of glycosylation and galactosylation of hydroxylysines. In the ER the collagen specific chaperone HSP47 binds to collagen III and plays key roles in collagen folding and transport to Golgi by binding TANGO1 and collagen. HSP47 is recycled from the Golgi back to the ER. In the Golgi and ECM, collagen III undergoes cleavage of the N- and C-propeptide, and the triple helical collagen III then forms heterotypic fibrils with collagen I.
