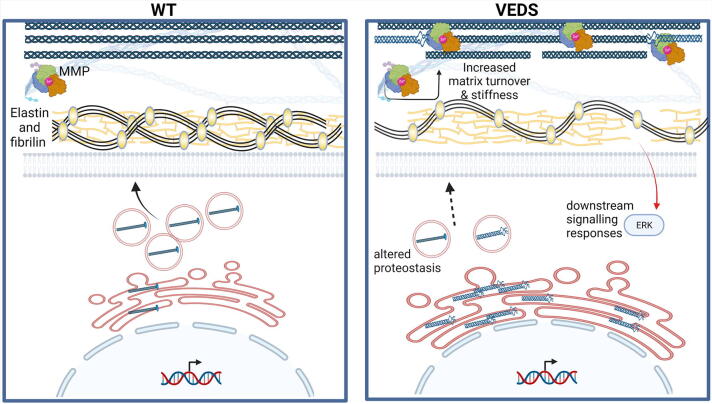
Fig. 2.
Potential disease mechanisms. Compared to wild type (WT), nonsense COL3A1 mutations lead to reduced secretion of wild type collagen III and extracellular levels of wild type collagen III causing fibrillar defects (interrupted fibrils). Missense mutations lead to expression of mutant collagen (distorted helix) that can be secreted and/or lead to intracellular accumulation of collagen in the ER, that can result in altered proteostasis indicated by ER enlargement. Therefore, both altered proteostasis and reduced secretion may occur simultaneously. The impact of COL3A1 mutations ion the ECM include reduced collagen III levels, and presence of mutant collagen III that may lead to disruption of the collagen network. This can be coupled with matrix turnover and degradation mediated, at least in part, by higher MMP levels. Furthermore, elastin defects occur with reduced levels of fibrillin 2. These ECM defects have been proposed to cause via an as yet unknown mechanism activation of ERK signalling. While ER enlargement is regularly observed, the nature of the altered proteostasis remains poorly defined. The multisystemic nature of vEDS also leaves the door open for cell and mutation specific effects, which represent an important gap in our knowledge.