FIG. 4.
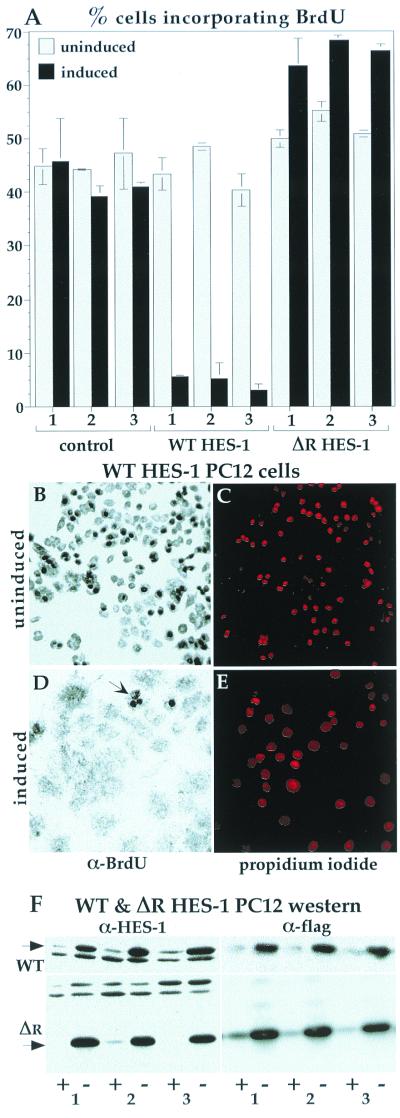
The inhibition of proliferation in HES-1-overexpressing cells was confirmed by BrdU incorporation. After 20 h of exposure to BrdU, about 40% of control cell lines had incorporated the BrdU nucleoside analogue into their DNA, as detected by anti-BrdU immunocytochemistry. This fraction of incorporation is consistent with the doubling time for this cell type of about 48 h. The level of incorporation for the three control lines was similar with or without induction of EGFP (A). The incorporation of BrdU into uninduced WT HES-1 PC12 cells was similar to control values. Upon induction of HES-1 (3 days prior to BrdU treatment), the level of BrdU incorporation dropped dramatically to about 5%, consistent with either a lower growth rate or a lower fraction of cells in S phase, and a lack of DNA synthesis (implied by the loss of PCNA). In contrast, induction of ΔR HES-1, a deletion mutant that lacks transcription repression activity (Fig. 7; discussed below) did not lower BrdU incorporation. Examples of the BrdU staining is shown for the WT HES-1 cells, both uninduced (B) and induced (D). Anti-BrdU staining is clearly seen as dark nuclei, which are mostly absent in the induced cells. The WT HES-1-induced cells that incorporated BrdU tended to have a rounded morphology (panel D, arrow), indicating either that they were in S phase or that they may have been low, or nonexpressing, subpopulations. The positions of the nuclei are shown by propidium iodide staining, which was partially obscured in the cells with high levels of BrdU incorporation due to the opacity of the DAB precipitate (C and E). (F) The induction of WT (F, upper panels) and ΔR HES-1 protein (F, lower panels) is shown by the anti-HES-1 and anti-Flag Western blots for three independent cell lines.