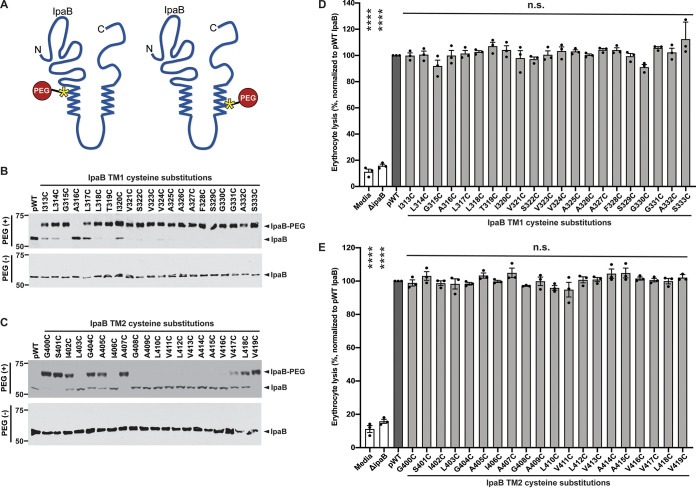
FIG 2.
Cysteine substitutions along IpaB are variably accessible in the context of soluble IpaB and do not impair pore formation in mammalian membranes. (A) Schematic showing PEG5000-maleimide labeling of soluble S. flexneri IpaB cysteine substitution derivatives. Transmembrane domains (zigzag lines), representative cysteine substitution derivatives along the length of IpaB (yellow asterisks), and PEG5000-maleimide (red circle) are depicted. (B and C) Gel migration of PEG5000-maleimide-labeled (IpaB-PEG) or unlabeled soluble IpaB in culture supernatants of indicated strains following chemical activation of type 3 secretion with Congo red. S. flexneri ΔipaB expressing wild-type IpaB (pWT) or a single IpaB cysteine substitution derivative was studied. Representative Western blots are shown. (D and E) Quantification of hemoglobin release by S. flexneri ΔipaB expressing wild-type IpaB (pWT) or a single cysteine substitution derivative. The abundance of hemoglobin release was quantified at A570 from at least three independent experiments. Means ± SEM are plotted. Black dots represent values obtained from individual experiments. The value for each cysteine substitution mutant to that of S. flexneri ΔipaB producing WT IpaB (pWT) was compared by ANOVA with Dunnett’s post hoc test and indicated as follows: ****, P < 0.0001; n.s., not significant. The positions of size markers (in kilodaltons) are shown to the left of the gels.