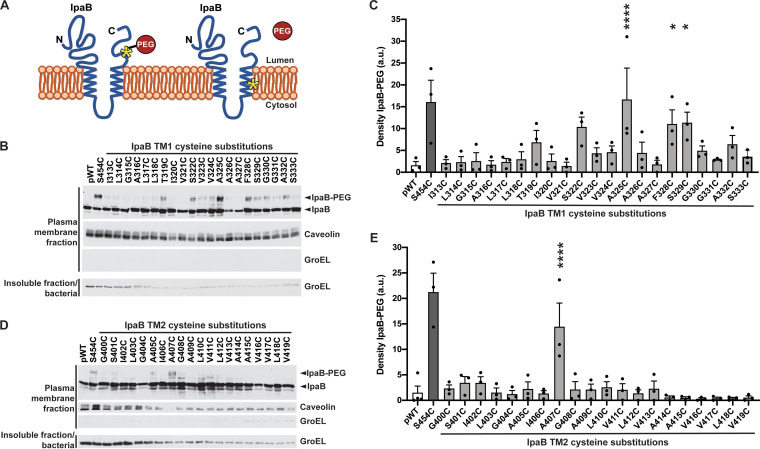
FIG 3.
Cysteine accessibility of transmembrane domains in membrane-embedded IpaB. (A) Schematic of IpaB topology mapping by PEG5000-maleimide labeling. The N- and C-terminal domains of IpaB are situated on the extracellular side of the plasma membrane. Cysteine substitutions are represented by an asterisk. PEG5000-maleimide is membrane impermeant and size excluded from passing all the way through the translocon pore; therefore, it does not react with cysteines on the cytosolic side of the plasma membrane or with cysteines facing the lipid bilayer. (B and D) Gel migration of PEG5000-maleimide-labeled (IpaB-PEG) or unlabeled IpaB in membrane- inserted translocons. S. flexneri ΔipaB expressing wild-type IpaB (pWT) or a single IpaB cysteine substitution derivative was studied. The positive control was IpaB S454, which lies in the C-terminal extracellular domain; S454C is accessible to PEG5000-maleimide labeling. Representative Western blots are shown. Caveolin-1, plasma membrane marker; GroEL, bacterial cytosolic protein. (C and E) Densitometry analysis of IpaB-PEG5000 bands from three independent experiments represented in panels B and D. The density is shown in arbitrary units (a.u.). Means ± SEM are plotted. Black dots represent values obtained from individual experiments. *, P < 0.05; ****, P < 0.0001; ANOVA with Dunnett’s post hoc test comparing IpaB labeling efficiency of S. flexneri ΔipaB expressing each IpaB cysteine derivative to S. flexneri ΔipaB expressing WT IpaB (pWT); the differences between these strains are not significant.