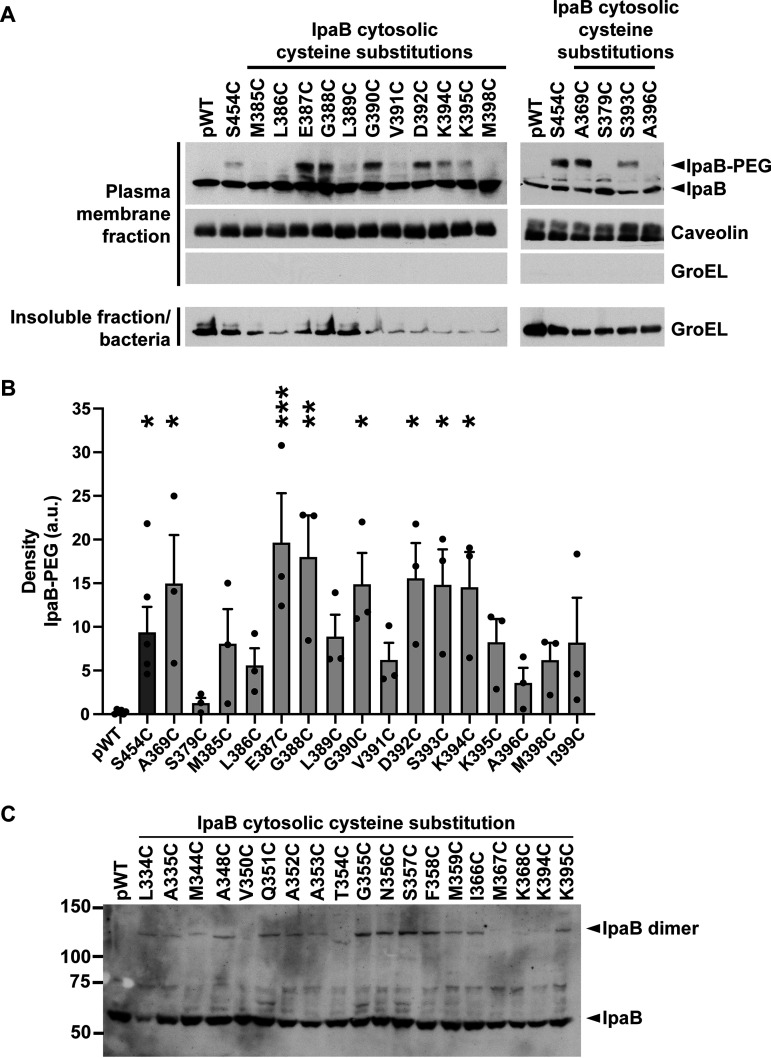
FIG 5.
When in membrane-embedded translocons, the cytosolic domain of IpaB is accessible to PEG5000 from the extracellular side of the plasma membrane. (A) Gel shift of PEG5000-maleimide-labeled, membrane-embedded IpaB from S. flexneri ΔipaB expressing wild-type IpaB (pWT) or a single IpaB cysteine substitution derivative. The positive control was IpaB S454, which lies in the C-terminal extracellular domain; S454C is accessible to PEG5000-maleimide labeling. Representative Western blots are shown. The positions of PEG5000-maleimide-labeled IpaB (IpaB-PEG), unlabeled IpaB, caveolin-1, a eukaryotic membrane marker, and GroEL, a bacterial cytosolic protein, are shown to the right of the gel. (B) Densitometry analysis of IpaB-PEG5000 bands from three independent experiments, represented in panel A. Means ± SEM are plotted. Black dots represent values obtained from individual experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ANOVA with Dunnett’s post hoc test comparing IpaB labeling efficiency of S. flexneri ΔipaB expressing each IpaB cysteine derivative to S. flexneri ΔipaB expressing WT IpaB (pWT). (C) Gel shift of copper 1,10-phenanthroline cross-linking of membrane-inserted IpaB. Representative Western blots are shown. The positions of IpaB (IpaB) and cross-linked IpaB (IpaB dimer) are shown to the right of the gel. The positions of size markers (in kilodaltons) are shown to the left of the gel.