FIG 2.
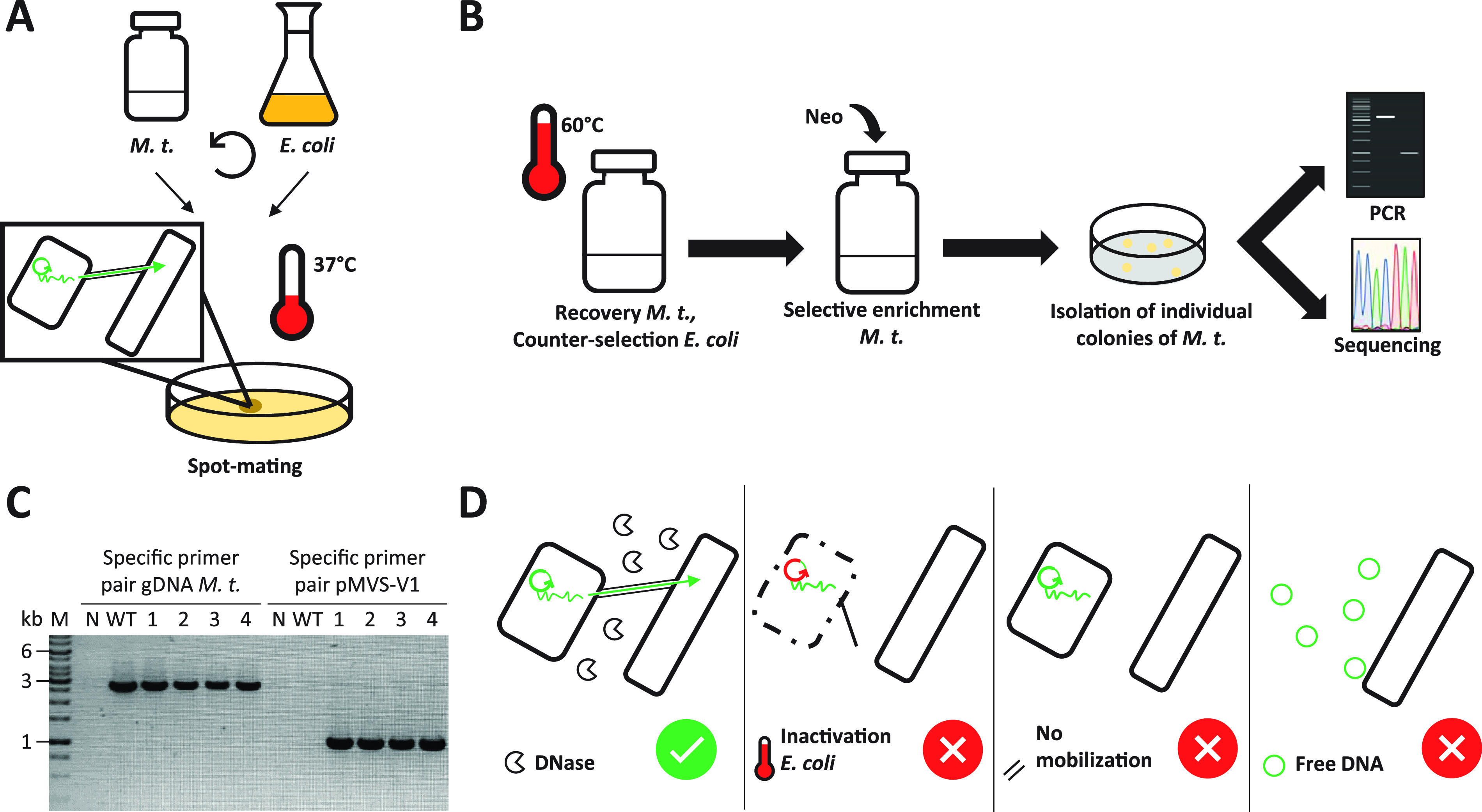
Schematic depiction and analysis of interdomain conjugation between E. coli S17-1 and M. thermautotrophicus ΔH. (A) Wild-type M. thermautotrophicus ΔH (M. t.) and the shuttle vector-carrying E. coli were harvested by centrifugation, mixed, and spotted on solidified medium plates that support growth of both microbes. During the spot-mating step at 37°C, the DNA transfer process via conjugation takes place (small scheme). (B) The process to isolate and identify individual colonies of genetically modified M. thermautotrophicus ΔH in the standard protocol. After the spot-mating step, M. thermautotrophicus ΔH cells were recovered in nonselective liquid mineral medium at 60°C, and afterward, transconjugants were enriched in neomycin (Neo)-containing selective liquid mineral medium at 60°C. Individual colonies were obtained from plating the enrichment culture. Those colonies were analyzed by PCR and Sanger sequencing. (C) PCR analysis of four respective transconjugants (1 to 4) with primer combinations specific for the shuttle vector pMVS-V1 replicon (1-kb fragment) and for genomic DNA (gDNA) of M. thermautotrophicus ΔH (2.8-kb fragment). N, water negative control; WT, control with wild-type M. thermautotrophicus ΔH; M, GeneRuler 1-kb DNA ladder (Thermo Scientific, Waltham, MA, USA). (D) Experimental conditions for the confirmation of conjugation as the mechanism for DNA transfer were (from left to right) DNase I treatment, heat inactivation of E. coli S17-1, conjugation with nonconjugative E. coli NEB stable, and addition of free plasmid DNA directly to M. thermautotrophicus ΔH cell culture.
