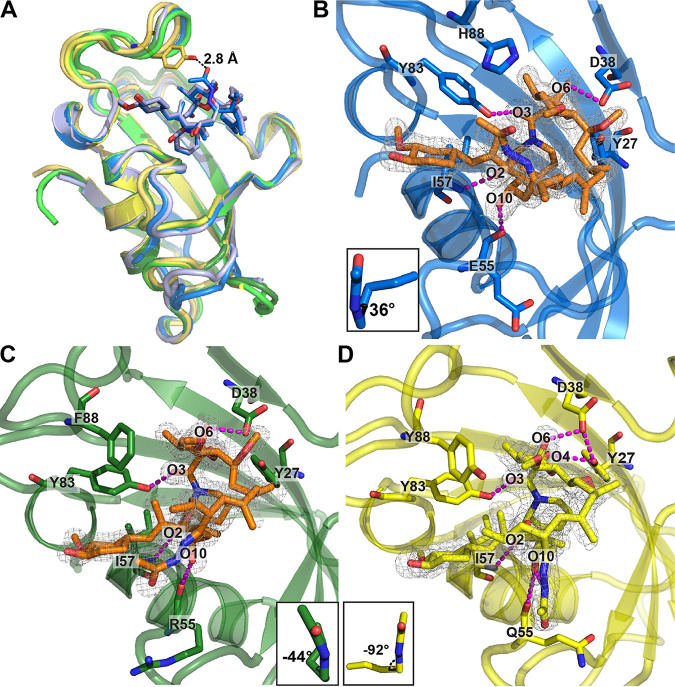
FIG 4.
Crystal structures of the human, A. fumigatus, and M. circinelloides FKBP12 proteins bound to APX879. (A) Comparison of the overlaid crystal structures of McFKBP12-APX879 (yellow; PDB ID 6VCT), McFKBP12-FK506 (gold; PDB ID 6VRX), hFKBP12-APX879 (blue; PDB ID 6VCU), hFKBP12-FK506 (light blue; PDB ID 1FKJ), AfFKBP12-APX879 (dark green; PDB ID 6VCV), and AfFKBP12(P90G)-FK506 (light green; PDB ID 5HWC). APX879 and FK506 from the hFKBP12 crystal structures are represented in blue and light blue sticks, respectively. Distance to APX879-C60 and McFKBP12-Tyr88 was estimated at 2.9 Å. (B to D) Representation of APX879 (orange) in the 2mFo-dFc density map (at 1 σ level) in the binding cavity of hFKBP12 (B), AfFKBP12 (C), and McFKBP12 (D). The C21-C22 dihedral angles measured are illustrated in the lower corner. Residues (Tyr27, Asp38, Eh/RAf/QMc55, Ile57, and Tyr83) are forming H-bonds (identified by magenta dashed lines) maintaining APX879 in the binding pockets. Table 1 presents data collection and refinement statistics.