Abstract
As the Fire Service becomes more aware of the potential health effects from occupational exposure to hazardous contaminants, personal protective equipment (PPE) manufacturers and fire departments have responded by developing and implementing improved means of firefighter protection, including more frequent laundering of PPE after exposures. While laboratory testing of new PPE designs and the effect of laundering on PPE fabric provides a useful way to evaluate these approaches, laboratory scale testing does not necessarily translate to full garment protection. Utilizing a fireground smoke exposure simulator, along with air and/or filter-substrate sampling for polycyclic aromatic hydrocarbons (PAHs) and benzene, this pilot study tested the chemical protective capabilities of firefighting PPE of different designs (knit hood vs particulate-blocking hood, turnout jacket with zipper closure vs hook & dee closure), including the impact of repeatedly exposing and cleaning (through laundering or decontamination on-scene) PPE 40 times. Overall, PAH contamination on filters under hoods in the neck region were higher (median PAHs = 14.7 μg) than samples taken under jackets in the chest region (median PAHs = 7.05 μg). PAH levels measured under particulate-blocking hoods were lower than levels found under knit hoods. Similarly, zippered closures were found to provide a greater reduction in PAHs compared to hook & dee closures. However, neither design element completely eliminated contaminant ingress. Measurements for benzene under turnout jackets were similar to ambient chamber air concentrations, indicating little to no attenuation from the PPE. The effect of laundering or on-scene decontamination on contaminant breakthrough appeared to depend on the type of contaminant. Benzene breakthrough was negatively associated with laundering, while PAHs were positively associated. More research is needed to identify PPE features that reduce breakthrough, how targeted changes impact exposures, and how fireground exposures relate to biological absorption of contaminants.
Keywords: firefighters, fireground, combustion products, occupational exposure, personal protective equipment (PPE), polycyclic aromatic hydrocarbons (PAHs)
INTRODUCTION
Structural fires fueled by common household furnishings can produce hundreds of compounds including those that may expose firefighters to vapor, gas and particulate contamination (Austin et al. 2001a, 2001b; Jankovic et al. 1991). Epidemiological studies suggest that firefighters have increased risk for numerous types of cancer including testicular, prostate, and non-Hodgkin’s lymphoma (IARC. 2010, Daniels et al. 2014, 2015; Glass et al. 2014; Jalilian et al. 2019; LeMasters et al. 2006; Lee et al.,2020; Pukkala et al. 2009; Tsai et al. 2015). As the fire service has become more aware of the fireground risks, personal protective equipment (PPE) manufacturers have developed new designs for turnout jacket and pants, hoods, gloves, and other aspects of the firefighting PPE ensemble. Fire departments have also become more consistent in deconning and/or laundering their PPE after exposures.
Dermal exposure is an important exposure pathway for firefighters (Stec et al. 2018; Fent et al. 2013), as studies have shown that PAHs (Vanrooij et al. 1993a, 1993b, 1994; Brzeznicki et al. 1997) and volatile organic compounds (VOCs) (Franz. 1984; Morgan et al. 1991; Wester and Maibach. 2000) can be absorbed through skin. Only a few studies have examined the penetration of fireground contaminants to the interior of the firefighting PPE ensemble. PAH concentrations measured under turnout gear have been 12–146 times lower than concentrations measured outside gear (Kirk and Logan. 2015; Wingfors et a. 2018). Other studies have investigated the ingress of contaminants through turnout gear by measuring the less volatile PAHs (those most likely to exist as particulate) on firefighters’ skin. Although there is variability among studies, there are apparent vulnerabilities in the neck region (Fent et al. 2014) and hands (Fent et al. 2017).
The firefighting PPE industry has rapidly developed and introduced new designs for the structural firefighting turnout ensemble that include new combinations of hood materials and smaller/tighter gaps or interfaces between each element of the PPE ensemble. The effectiveness of these interventions has largely been limited to laboratory scale testing and/or anecdotal evidence, as there has been only limited testing with full ensembles under realistic fireground conditions.
Recent studies have also focused on the effectiveness of post-fire cleaning of PPE to remove contamination (Fent et al. 2017; Keir et al. 2020). Updates to selection, care and maintenance standards such as NFPA 1851 have highlighted the critical nature of post-fire contamination reduction (NFPA. 2020). Yet, the impact of repeated cleaning of the gear on the level of protection from fire smoke contamination is not known.
This pilot study sought to determine how 40 repeated exposures and cleanings (laundering and on-scene decontamination) impacted the ingress of PAH and benzene contamination for different hood designs (traditional knit vs particulate-blocking) and turnout jacket closure systems (zipper vs hook & dee) in NFPA 1971 (NFPA. 2018) compliant PPE.
METHODS
Fireground Exposure Simulator (FES)
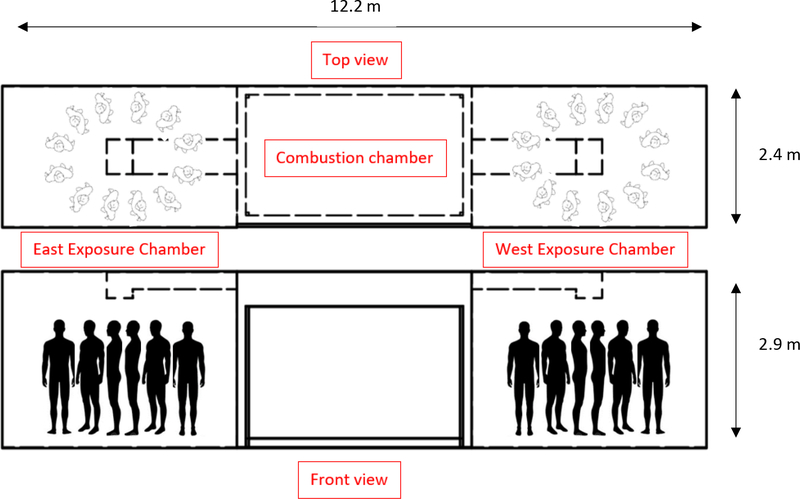
The Fireground Exposure Simulator (FES) was developed from a steel intermodal shipping container, with the middle section serving as a combustion chamber generated by burning a commercially available sofa, and fire effluent ducted into two exposure chambers with up to 12 full-sized mannequins in each chamber (Figure 1) (Horn et al. 2020). For this study, nine mannequins were dressed in full PPE ensembles including different jacket closures (zipper vs. hook & dee) and hood designs (knit vs. particulate-blocking), placed in one (east) of the two chambers, and exposed to smoke from a burning sofa for a total of 10 minutes per exposure trial (40 trials). The timing of ignition, ventilation and suppression were the same for each exposure trial and were patterned after the interior attack scenario conducted during a previous fireground study (Horn et al. 2017). While important day-to-day variability exists when utilizing the FES, such variations are similar to those measured from a simulated fireground study (Horn et al. 2020). After each exposure trial, turnout jackets were cleaned either by laundering or on-scene decontamination. Ambient chamber and under PPE samples were collected from the east chamber during four of the exposure trials (1st, 10th, 20th, and 40th). The study design and sampling strategy is outlined in detail in Figure 2.
Figure 1.
Overhead and front view of Fireground Smoke Exposure prop with mannequin orientation (samples collected in the east exposure chamber only).
Figure 2.
Study design and sample collection strategy
Firefighting PPE
A total of nine new sets of structural firefighting PPE were produced to be worn by full-sized mannequins using popular market options consisting of standard outer shell, moisture barrier, and thermal layer. One set of turnout jackets were manufactured with zipper (n=6) and Velcro closures at the front of the jacket, while a second set (n=3) was produced using hook & dee closures to study the impact of the jacket closure system on ingress of fire effluent (Figure 3). Half of the mannequins wearing zippered jackets were dressed with a two layer Nomex® (DuPont) knit hood (n=3) and the other half with commercial Nomex® knit hood with a polymer barrier as a third interstitial layer (particulate-blocking hood; n=3).
Figure 3.
Side-by-side comparison of zipper and hook & dee turnout jacket closures.
PPE Cleaning
After each exposure trial, the zippered PPE ensembles received one of two cleaning treatments: (1) laundered (n=3) following manufacturer’s machine wash cleaning instructions in a front load washing machine without an agitator for a 55 minute wash/rinse cycle with detergent and dried in drying cabinet with forced air circulating at 105° F as outlined in NFPA 1851 guidelines (NFPA 2020) or (2) decontamination (n=3) using a wet soap method as described in Fent et al (2017). All ensembles produced with hook & dee closures (n=3) were laundered (separate from the zippered PPE). The three treatment groups (hook & dee /laundered, zipper/laundered, zipper/decon) went through 40 exposure and cleaning cycles in this study. To complete the PPE ensemble, an SCBA facepiece was put on each mannequin along with a hood. After each trial, all hoods were laundered following NFPA 1851 guidelines (NFPA 2020).
Ambient chamber and Under PPE Sampling
Ambient chamber and under PPE samples were collected during the 1st, 10th, 20th, and 40th exposure trials. Ambient chamber air samples (6 × 70-mm glass charcoal tubes and 13–8 X 75-mm glass OVS-XAD-7 tubes) were mounted on the outside of the PPE or on a tripod at chest height to determine the magnitude of combustion byproducts (VOCs and PAHs) in the atmosphere. We collected at least one ambient east chamber VOC and PAH air sample for each trial. Under PPE air samplers (charcoal tubes) were positioned in interior pockets of the jacket at lower rib height of the mannequins. Pumps were calibrated using a low or medium flow Drycal Defender (MesaLabs, Lakewood, CO). All air samples had post-calibration flow rates that were within 5% of the pre-calibration flow rate. Pre-calibration flow rates were based on the target flow rates of 1.0 L/min for OVS-XAD-7 and 0.1 L/min for charcoal tubes. One field blank was collected during each fire for each type of sampling media. After each trial, the samples were collected, capped, and stored in a freezer. The charcoal tubes were analyzed using NIOSH Method 1501 for BTEX (benzene, toluene, ethylbenzene, and xylenes) (NIOSH, 2013). The OVS-XAD-7 tubes were analyzed for PAHs using NIOSH Method 5506.
Air samples that did not run for at least three minutes of the fire were excluded because they may not accurately reflect the average concentration during the 10-minute scenario, consistent with previous controlled-fire evaluations (Fent et al. 2018; 2019). In total, three BTEX air samples were excluded due to a sampling time of less than three minutes. Because we collected replicates for samples, these exclusions were not expected to adversely affect our findings. All remaining BTEX samples and all PAH samples ran for at least 4 minutes of the fire scenario, and heavy smoke development generally occurred within the first 3–4 minutes. While the ambient air samples were stopped immediately after the mannequins were pulled from the structure, the under PPE air samples continued running within the enclosed gear for an undocumented amount of time. To account for this difference, the under PPE samples were adjusted by adding an estimated 2-minutes to their sampling time or duration of the fire scenario.
To evaluate the infiltration and impaction or deposition of particulate-phase PAHs through PPE and PPE interfaces, 90-mm PTFE filters were affixed to the mannequins’ necks under the hood and inner wrists (using lab tape) and mannequins’ chests (using alligator clips attached to the outside of a base layer t-shirt). Aluminum backing was placed on the PTFE filters attached to shirts to limit particulate loading to the outward facing side of the filter. The filter samples were intended to serve as surrogate skin or base-layer surfaces, and each had approximately 6.4 cm x 6.4 cm area available for particle flux. After each trial, the hoods and turnout jackets were carefully removed from the mannequins. Research staff then put on new nitrile gloves and collected the filters (removing aluminum backing and/or tape) into 50-mL opaque Falcon tubes, which were then stored in a freezer. The filters were analyzed for PAHs using NIOSH Method 5506 (NIOSH, 2013). The mannequins were thoroughly cleaned using alcohol wipes containing emulsifiers and detergents in between each exposure trial to minimize the potential for cross contamination.
Data Analysis
For each PAH sample, total PAHs were calculated by summing the concentrations (OVS-XAD-7 air samples = μg/m3; PTFE filters = μg/filter) of the 15 PAHs. Zero was used for non-detectable concentrations of individual PAHs in this summation (Fent et al. 2019). For each exposure trial, we calculated the median of the ambient PAH and benzene air concentrations. For exposure trials where only one ambient air sample was collected, the concentration for that sample was used in lieu of a median value for all comparisons. Because measurements under gear are presumably correlated with measurements outside of gear in the ambient air, we calculated the ratio of the median ambient air concentration (found in Table 1) to the levels measured under the gear, for both total PAHs and benzene. While the ambient and under-PPE PAH measurements have different units, these ratios provide a means of standardizing the results across the exposure trials where median air concentrations can vary due to typical and known variations in fire development. Benzene concentrations measurements under the turnout jacket were collected using the same techniques as the ambient air concentrations, so traditional workplace protection factors (WPFs) could be quantified. WPFs estimate the level of protection for a worker in a hazardous environment and is expressed as the concentration of a hazardous substance outside the PPE divided by the concentration inside the PPE. Higher WPFs indicate a higher level of protection.
Table 1.
Total PAH (μg/m3) and benzene (ppb) ambient east chamber air concentrations
| Analyte | Exposure Trial | N | Air median | Range | p-valueA |
|---|---|---|---|---|---|
| Total PAHs | New | 2 | 285,500 | 277,000 – 294,000 | 0.0312 |
| 10 | 2 | 225,500 | 213,000 – 238,000 | ||
| 20 | 2 | 157,500 | 136,000 – 179,000 | ||
| 40 | 3 | 70,700 | 40,000 – 125,000 | ||
| Benzene | New | 2 | 189,500 | 152,000 – 227,000 | 0.639 |
| 10 | 1 | 187,000 | N/A | ||
| 20 | 1 | 314,000 | N/A | ||
| 40 | 1 | 199,000 | N/A |
p-values calculated from ANOVA on the log of the ambient PAH and benzene air concentrations.
In all analyses, dependent variables were log-transformed due to data being right skewed. An ANOVA test was used to determine if there were differences in means between trials for the log of ambient PAH and the log of benzene air concentrations. To determine if a relationship existed between laundering cycles and protection factors, multiple linear regression analyses of log-transformed ratios of benzene and PAH concentrations under jackets to ambient air concentrations were performed. More precisely, to model the WPF of each sample, the regression formulas took the general form:
where Yi represents under gear sample (either total PAH or benzene), gear type is an indicator for type of closure (hook & dee or zipper) or type of hood (knit or particulate-blocking), β1 represents the change in the log of WPF per cleaning, and β2 represents the effect gear type. Analyses were performed in SAS 9.4 (Cary, NC). Summary results are expressed as median and range.
RESULTS
PAH Ingress
PAH concentrations were measured in the ambient chamber air (Table 1), under turnout jackets in the chest region (Table 2), and on the neck under hoods worn by mannequins (Table 3). Median PAH concentrations measured on PTFE filters from under the laundered hook & dee jackets in the chest region were 1.5-fold higher than those measured under the laundered zippered jackets. Over the 40 cycles, the ratio of PAH contamination under jackets in the chest region to ambient chamber air concentrations decreased for all three treatment groups (hook & dee /laundered, zipper/laundered, zipper/decon).
Table 2.
Median total PAH concentration (μg/filter) on PTFE filters under jackets (chest). Ratios calculated using median ambient chamber air PAH concentrations are also provided in parentheses to estimate protection factors.
| Laundered |
Decon |
||||||
|---|---|---|---|---|---|---|---|
| Hook |
Zipper |
Hook vs Zipper | Zipper |
||||
| Exposure Trial | N | Median Total PAH (RatioA) | N | Median Total PAH (RatioA) | p-valueB | N | Median Total PAH (Ratio) |
|
| |||||||
| New | 3 | 6.28 (45,500) | 2 | 7.07 (44,400) | 0.037 | 1 | 7.23 (39,500) |
| 10 | 3 | 15.6 (14,400) | 3 | 2.01 (112,400) | 3 | 4.46 (50,600) | |
| 20 | 2 | 11.5 (25,900) | 3 | 3.63 (43,400) | 3 | 7.34 (21,400) | |
| 40 | 1 | 7.68 (9,200) | 3 | 7.35 (9,600) | 3 | 5.53 (12,800) | |
|
| |||||||
| Trend P-valueC | 0.1 | 0.01 | 0.105 | ||||
To estimate the impact of repeated exposure and laundering on PPE protection factors, we calculated the ratio of median ambient chamber air PAH concentrations (table 1) to median PAH levels on PTFE filters under jackets (chest region).
P-value from multiple linear regression of the log-transformed ratios of PAHs deposited under jackets in the chest region to median ambient chamber air PAH concentrations by type of jacket closure, controlling for cleaning cycles/exposure trial
P-value from multiple linear regression of log-transformed ratios vs. cleaning cycles/exposure trial, controlling for type of jacket closure
Table 3.
Median total PAH concentration (μg/filter) on PTFE filters under laundered hoods. Ratios calculated using median ambient chamber air PAH concentrations are also provided in parentheses to estimate protection factors.
| Knit |
Particulate-Blocking |
Knit vs Particulate Blocking | |||
|---|---|---|---|---|---|
| Exposure Trial | N | Median Total PAH (RatioA) | N | Median Total PAH (RatioA) | p-valueB |
|
| |||||
| New | 3 | 26.3 (10,900) | 3 | 16.2 (17,600) | 0.003 |
| 10 | 3 | 18.3 (12,300) | 3 | 13.1 (17,200) | |
| 20 | 3 | 17.8 (8,800) | 3 | 11.4 (13,800) | |
| 40 | 3 | 13.0 (5,500) | 3 | 6.65 (10,600) | |
|
| |||||
| Trend P-valueC | 0.001 | 0.045 | |||
To estimate the impact of repeated exposure and laundering on PPE protection factors, we calculated the ratio of median ambient chamber air PAH concentrations (table 1) to median PAH levels on PTFE filters under hoods (neck region)
P-value from multiple linear regression of the log-transformed ratios of PAHs deposited under jackets in the chest region to median ambient chamber air PAH concentrations by type of hood, controlling for cleaning cycles/exposure trial
P-value from multiple linear regression of the log-transformed ratios vs. cleaning cycles/exposure trial, controlling for type of hood
PTFE filters under particulate-blocking hoods collected about 30% less PAHs than the same measurements under the knit hoods. The ratio of PAH contamination under hoods to ambient chamber air concentrations decreased over the 40 trials for both types of hoods. PTFE filters were also placed in the wrist region of the mannequins, but all results were below or near the limit of detection (LOD < 4.7 μg/filter).
Benzene Ingress and Effect of Turnout Jacket Closure
Overall, ambient chamber benzene concentrations (Table 1) and the concentrations in the air under both types of turnout jackets were similar (Table 4). Toluene, ethylbenzene, and xylene concentrations were slightly higher under turnout jackets with hook & dee closures compared to zippered closures (Supplemental Materials, S1).
Table 4.
Median benzene concentration (ppb) under jackets. Ratios calculated using median ambient chamber air benzene concentrations are also provided in parentheses to calculate workplace protection factors (WPFs).
| Laundered |
Decon |
||||||
|---|---|---|---|---|---|---|---|
| Hook |
Zipper |
Hook vs Zipper |
Zipper |
||||
| Exposure Trial |
N |
Median Benzene (RatioA) |
N |
Median Benzene (RatioA) |
p-valueB | N |
Median Benzene (Ratio) |
| New | 3 | 348,000 (0.54) | 3 | 357,000 (0.53) | 0.462 | 2 | 282,000 (0.7) |
| 10 | 3 | 251,000 (0.74) | 3 | 213,000 (0.88) | 2 | 162,000 (1.16) | |
| 20 | 1 | 213,000 (1.47) | 2 | 248,000 (1.29) | 2 | 214,000 (1.48) | |
| 40 | 1 | 137,000 (1.45) | 1 | 150,000 (1.32) | 2 | 115,000 (1.73) | |
|
| |||||||
| Trend P-valueC | 0.017 | 0.003 | 0.008 | ||||
To estimate the impact of repeated exposure and laundering on PPE protection factors, we calculated the ratio of median ambient chamber air benzene concentrations (table 1) to benzene under jackets (rib region)
P-value from multiple linear regression of the log-transformed ratios of benzene under jackets in the chest region to median ambient chamber air benzene concentrations by type of jacket closure, controlling for cleaning cycles/exposure trial
P-value from multiple linear regression of the log-transformed ratios vs. cleaning cycles/exposure trial, controlling for type of jacket closure
The most surprising finding from this study was the high percentage of benzene that was shown to accumulate in the interior space of the protective ensemble worn by the mannequins compared to the ambient concentrations (Table 4). Overall, benzene WPFs increased for all three treatment groups (hook & dee /laundered, zipper/laundered, and zipper/decon) over the 40 cycles.
DISCUSSION
This pilot study evaluated protection provided by PPE worn by the mannequins with different jacket closures and hood designs that were repeatedly exposed and laundered or decontaminated up to 40 times. Though our sample size for each trial is small, these results provide the first quantifiable data of the difference in possible protection from PAH ingress between hook & dee and zipper closures. The hook & dee closure system leaves multiple open paths where fire effluent can flow into the jacket around the individual clasps, which are not present for the zippered closure. Thus, it is not surprising that the hook & dee style gear resulted in higher penetration of PAHs that were captured on the PTFE filters in the chest region of the mannequins.
Across the four trials presented in this study, PAH contamination under knit hoods worn by mannequins was consistently higher than PAH contamination found under particulate-blocking hoods. While these differences show a meaningful reduction in the contamination that could potentially reach firefighters’ skin, it is notable that PAHs were still detected in the neck region under the particulate-blocking hoods worn by the mannequins on all 12 PTFE filter samples analyzed in this study.
The ambient chamber PAH air concentrations in this study (range in medians: 70,700 – 285,500 μg/m3) were generally higher than levels reported in our previous residential fire and training fire studies (maximum 78,200 μg/m3) (Fent et al. 2018, 2019). This relative increase in concentration may provide a high-concentration challenge to identify possible leakage paths in the PPE, which is similar to traditional laboratory simulant tests (Horn et al. 2020). While it is likely that the magnitude of particle penetration (mass flux) is related to the ambient chamber air concentration (and hence the rationale for expressing results as PAH ratios in this manuscript), it is also possible that this pathway could become saturated, whereby higher ambient concentrations may not necessarily translate into higher flux. When ambient air has high concentrations of PAHs, the amount flowing through interfaces in the jacket may reach a maximum capacity. Thus, the lower protection factors (PAH ratios) consistently observed after 40 laundering/decontamination cycles in Tables 2–3 could be artifacts that resulted from the relatively lower PAH concentrations in ambient air during that trial. In other words, while the ambient concentration dropped, the flux may have remained constant. However, further examination is required and caution should be exercised in interpreting these findings.
Overall, benzene concentrations in the ambient chamber air (range: 199,000 – 314,000 ppb) and under gear (range in medians: 115,000 – 357,000 ppb) in this study were similar to the maximum personal concentrations reported previously for attack firefighters by Fent et al. (2018) (322,000 ppb), but were higher than the maximum concentrations reported by Austin et al. in 2001 (11,000 ppb) and Jankovic et al. in 1991 (22,000 ppb) (Austin et al. 2001B; Jankovic et al. 1991; Fent et al. 2018). The higher exposure burden to benzene here may be due to the mannequins being in standing position and exposed to the upper gas layer. It is also important to note that the turnout gear remained zipped-up for several minutes after exiting the structure. The under-gear sampling times were adjusted by 2-minutes to account for this difference, but it is possible, especially in the early stages of the study, that it took more than 2-minutes to remove and stop the under-gear samples. This likely explains why WPFs were <1 for the new and 10x laundered gear.
With this limitation in mind, the apparent increasing WPFs seen with repeated exposure/cleaning cycle may be an artifact of the investigators becoming more efficient in removing and stopping the under-gear samples once the mannequins were pulled from the structure in the later stages of the study. It is also possible that the softening of the turnout gear textiles with repeated laundering led to tighter fit on the mannequins, which slightly retarded the infiltration of the vapors. However, the PAH penetration data does not appear to support this theory. Another possibility is that the softening of the textiles resulted in more adsorption of benzene (due to increased surface area and diffusion) thereby reducing the amount available for penetration. Further research is necessary to fully understand these findings.
We deliberately did not conduct air sampling for PAHs under turnout gear because a higher flow rate common with this type of sampling (≥1 L/min) would have introduced a potential bias by drawing contaminants into and through the gear. This means that the data collected here cannot be used to calculate traditional WPFs for the PAHs. Kirk and Logan (2015) estimated an average WPF for total PAHs of 12. The WPFs estimated for benzene in this study (< 1.0 – 1.7) indicate a much higher breakthrough for more volatile compounds. Gas molecules such as benzene can more readily penetrate the small gaps in PPE interfaces than the particulate contamination (i.e., most PAHs). Naphthalene is the most volatile PAH and it is likely that it had a higher breakthrough relative to the other PAHs, just as Wingfors et al. (2018) had previously shown. Naphthalene was the most abundant PAH measured, contributing over 75% of the total PAHs quantified on PTFE filters under hoods and 54% on filters under jackets (Supplemental Materials, S2). Note, however, that because naphthalene is volatile, the filter samples likely underestimated the amount penetrating the gear.
Taken together, these findings highlight the complex pathways leading to dermal exposure of both non-volatile and volatile compounds. Higher transdermal absorption of naphthalene, benzene, and other volatile compounds can be expected the longer skin is exposed. For example, Franz (1984) found higher absorption of benzene when trapped against the skin rather than allowing for evaporation. Moisture on the skin, which is inevitable for firefighters working in fully encapsulating turnout gear, may also facilitate absorption of water-soluble compounds like benzene (Franz. 1984).
These results suggest that firefighters may be able to reduce the magnitude of dermal absorption for volatile compounds by more quickly unzipping and airing out their turnout gear after firefighting, although additional study is warranted to test this hypothesis. Firefighters should continue cleaning their skin with wipes and showering as quickly as possible post-fire. The trends in protection between the three turnout jacket groups (hook & dee /laundered, zipper/laundered, and zipper/decon) were similar over the 40 treatment cycles. Overall, the fireground particulate protection capability of turnout gear does not appear to be negatively impacted by laundering or on-scene decontamination for up to 40 cycles. Additional PPE design evolution should be directed at reducing vapor penetration to compliment the current design evolution focused on reducing particulate penetration. Because our sample sizes for each trial were small, validation of the findings presented here through additional tests would be beneficial.
This study had other limitations in addition to small sample sizes. Temporal and spatial variability in combustion products is expected within the FES chambers, as seen by the range in median PAH air concentrations between trials (70,700 −285,500 μg/m3). This known variability in test conditions is similar to that measured during simulated residential fires and can be both a benefit and a limitation of the technique (Horn et al 2020). Ambient chamber air samples were used in an effort to account for this temporal variability, although it is possible that there was a saturation effect for PAHs at higher concentrations. It is also possible that active sampling of benzene under the gear created a gradient to draw in contaminants. However, the air flows were purposely kept low (0.1 L/min) to move less air (~1 liter) than is naturally present on the interior of the ensemble. Another limitation is that we did not sample the air under turnout gear for naphthalene or other PAHs, which could have provided additional data to explore chemical breakthrough. Additionally, the replacement of non-detects with zero may underestimate the total PAH concentration for samples taken in this study. However, we believe this is more appropriate than using other non-detect replacement methods which may overestimate the magnitude of total PAHs. Note that most of the individual PAHs were detectable in the air samples. So, replacement of non-detects with zero would mostly affect the under-gear samples. Hence, any biases with this approach would likely results in an underestimation of the PAH penetration through turnout gear and/or hoods.
CONCLUSIONS
Combustion products penetrated different components of the turnout gear at varying magnitudes. Particulate-blocking hoods reduced PAHs reaching the mannequins’ necks by more than 30% compared to knit hoods. However, particulate-blocking hoods did not completely eliminate the presence of PAHs in the neck region of the mannequins. Median PAH concentrations under hook & dee style closures were 1.5-fold higher than zippered closures, while benzene was shown to readily breakthrough the turnout gear irrespective of the style of jacket closure, and in some cases samples taken from under the jacket were actually higher than the ambient chamber air concentrations. The effect of laundering and on-scene decontamination on the chemical-protective properties of the turnout gear appeared to depend on the type of combustion products evaluated in this study; although, variability in environmental conditions could also play a role in these findings. Further research is needed to confirm these findings.
Supplementary Material
Acknowledgements
This was a collaborative project that could not have been completed without the help from several people. We thank Kenneth Sparks for preparing and maintaining our sampling equipment. This study was approved by the Institutional Review Boards at NIOSH and the University of Illinois. This work was supported by the Department of Homeland Security Fire Prevention and Safety Grant #EMW-2015-FP-00646. The findings and conclusions are those of the author(s) and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
REFERENCES
- Austin CC, Wang D, Ecobichon D, Dussault G. 2001a. Characterization of volatile organic compounds in smoke at experimental fires. J Toxicol Environ Health A. 63(3): 191–206. [DOI] [PubMed] [Google Scholar]
- Austin CC, Wang D, Ecobichon D, Dussault G. 2001b. Characterization of volatile organic compounds in smoke at municipal structural fires. J Toxicol Environ Health A, 63(6): 437–58 (2001). [DOI] [PubMed] [Google Scholar]
- Brzeznicki S, Jakubowski M, and Czerski B. 1997. Elimination of 1-hydroxypyrene after human volunteer exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health 70(4): 257–60. [DOI] [PubMed] [Google Scholar]
- Daniels RD, Kubale T, Yiin J. et al. 2014. Mortality and cancer incidence in a pooled cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occup Environ Med. 71(6): 388–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RD, Bertke S, Dahm M, Yiin J, Kubale T, Hales T, et al. 2015. Exposure–response relationships for select cancer and non-cancer health outcomes in a cohort of US firefighters from San Francisco, Chicago and Philadelphia (1950–2009). Occupational and Environmental Medicine 72(10):699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Eisenberg J, Evans D, Sammons D, Robertson S, et al. 2013. Evaluation of Dermal Exposure to Polycyclic Aromatic Hydrocarbons in Fire Fighters. U.S. Department of Health and Human Services. [Google Scholar]
- Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil J, Stiegel M. 2014. Systemic Exposure to PAHs and Benzene in Firefighters Suppressing Controlled Structure Fires. The Annals of Occupational Hygiene 58(7):830–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fent KW, Alexander B, Roberts J Roberts. Robertson S, Toennis C, Sammons D et al. 2017. Contamination of firefighter personal protective equipment and skin and the effectiveness of decontamination procedures. J. Occup. Environ. Hyg. 14(10): 801–814. [DOI] [PubMed] [Google Scholar]
- Fent KW, Evans D, Babik K, Striley C, Bertke S et al. 2018. Airborne contaminants during controlled residential fires. J. Occup. Environ. Hyg. 15(5): 399–412. [DOI] [PubMed] [Google Scholar]
- Fent KW, Mayer A, Bertke S, Kerber S, Smith D. et al. 2019. Understanding airborne contaminants produced by different fuel packages during training fires. J Occup Environ Hyg. 16(8): 532–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz TJ. 1984. Percutaneous absorption of benzene, in Advances in Modern Environmental Toxicology. Vol. 6, Applied Toxicology of Petroleum Hydrocarbons, McFarland HN, Editor. Scientific Publishers: Princeton, NJ. 61–70. [Google Scholar]
- Glass D, Sim M, Pircher S, Del Monaco A, Dimitriadis C, Miosge J. 2014. Final Report Australian Firefighters’ Health Study January 3, 2017; Available from: http://www.coeh.monash.org/downloads/finalreport2014.pdf.
- Horn GP, Kesler RM, Kerber S, Fent KW, Schroeder TJ, Scott WS, et al. 2017. Thermal response to firefighting activities in residential structure fires: impact of job assignment and suppression tactic. Ergonomics 61(3):404–419. [DOI] [PubMed] [Google Scholar]
- Horn GP, Kerber S, Lattz J, Kesler RM, Smith DL, et al. 2020. Fireground Exposure Simulator (FES) for Fireground Smoke and Heat Intervention Testing. Fire Technology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC). 2010. Painting, Firefighting, and Shiftwork, in IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 98. Lyon, France: World Health Organization. [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, Jones W, Burkhart J, Noonan G. 1991. Environmental study of firefighters. Ann. Occup. Hyg. 35(6): 581–602. [DOI] [PubMed] [Google Scholar]
- Jalilian HZ,M; Weiderpass E; Rueegg C; Khosravi Y; Kjaerheim K 2019. Cancer incidence and mortality among firefighters. Int J Cancer, 145:2639–2646. [DOI] [PubMed] [Google Scholar]
- Keir JLA, Akhtar U, Matschke D, White P, Kirkham T, et al. 2020. Polycyclic aromatic hydrocarbon (PAH) and metal contamiation of air and surfaces exposed to combustion emmisions during emergency fire suppression: Implications for firefighters’ exposures. Total Environ. 698. [DOI] [PubMed] [Google Scholar]
- Kirk KM and Logan MB. 2015. Firefighting instructors’ exposures to polycyclic aromatic hydrocarbons during live fire training scenarios. J. Occup. Environ. Hyg. 12(4): 227–234. [DOI] [PubMed] [Google Scholar]
- Lee DJ, Koru-Sengul T, Hernandez MN, Caban-Martinez AJ, McClure LA, Mackinnon JA, & Kobetz EN (2020). Cancer risk among career male and female Florida firefighters: Evidence from the Florida Firefighter Cancer Registry (1981–2014). American Journal of Industrial Medicine. [DOI] [PubMed] [Google Scholar]
- LeMasters GK; Genaidy AM; Succop P; Deddens J; Sobeih T; Barriera-Viruet H; Dunning K; Lockey J 2006. Cancer risk among firefighters: a review and meta-analysis of 32 studies. J Occup Environ Med, 48: 1189–1202. [DOI] [PubMed] [Google Scholar]
- Morgan D, Cooper S, Carlock D, Sykora J, Sutton B, et al. 1991. Dermal absorption of neat and aqueous volatile organic chemicals in the fischer 344 rat. Environmental Research. 55(1): 51–63. [DOI] [PubMed] [Google Scholar]
- National Fire Protection Association (NFPA). 2018. NFPA 1971 standard on protective ensembles for structural fire fighting and proximity fire fighting. National Fire Protection Association: Quincy, MA. [Google Scholar]
- National Fire Protection Association (NFPA). 2020. NFPA 1851 Standarn on Selection, Care, and Maintenance of Protective Ensembles for Structural Fire Fighting and Proximity Fire Fighting. National Fire Protection Association; Quincy, MA. [Google Scholar]
- National Institute for Occupational Safety and Health (NIOSH). 2013. Manual of analytical methods 4th ed Publication No. 94–113 (August 1994); 1st Supplement Publication 96–135, 2nd Supplement Publication 98–119, 3rd Supplement Publication 2003–154. U.S. Department of Health and Human Services: Cincinnati, OH. [Google Scholar]
- Pukkala E, Martinesen J, Lynge E, Gunnarsdottir H, Sparen P, Tryggvadottir L, et al. 2009. Occupation and cancer - follow-up of 15 million people in five Nordic countries. Acta Oncologica 48(5): 646–790. [DOI] [PubMed] [Google Scholar]
- Stec AA, Dickens K, Salden M, Hewitt F, Watts D, et al. 2018. Occupational Exposure to Polycyclic Aromatic Hydrocarbons and Elevated Cancer Incidence in Firefighters. Sci Rep 8(1): 2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai RJ, Luckhaupt S, Schumacher P, Cress R, Deapen D, Calvert G. 2015. Risk of cancer among firefighters in California, 1988–2007. Am J Ind Med. 58(7): 715–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRooij JG, Bodelier-Bade M, Jongeneelen F. 1993a. Estimation of individual dermal and respiratory uptake of polycyclic aromatic hydrocarbons in 12 coke oven workers. British Journal of Industrial Medicine 50(7): 623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanRooij JG, De Roos JH, Bodelier-Bade M, Jongeneelen F. 1993b. Absorption of polycyclic aromatic hydrocarbons through human skin: differences between anatomical sites and individuals’. J Toxicol Envl Health 38: 355–68. [DOI] [PubMed] [Google Scholar]
- VanRooij JG, Bodelier-Bade M, Hopmans P, Jongeneelen F. 1994. Reduction of urinary 1-hydroxypyrene excretion in coke-oven workers exposed to polycyclic aromatic hydrocarbons due to improved hygienic skin protective measures. Annals of Occupational Hygiene. 38(3): 247–56. [DOI] [PubMed] [Google Scholar]
- Wingfors H, Nyholm J, Magnusson R, Wijkmark C. 2018. Impact of Fire Suit Ensembles on Firefighter PAH Exposures as Assessed by Skin Deposition and Urinary Biomarkers. Ann Work Expo Health. 62(2): 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester RC and Maibach HI. 2000. Benzene percutaneous absorption: dermal exposure relative to other benzene sources. International Journal of Occupational and Environmental Health. 6(2): 122–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.