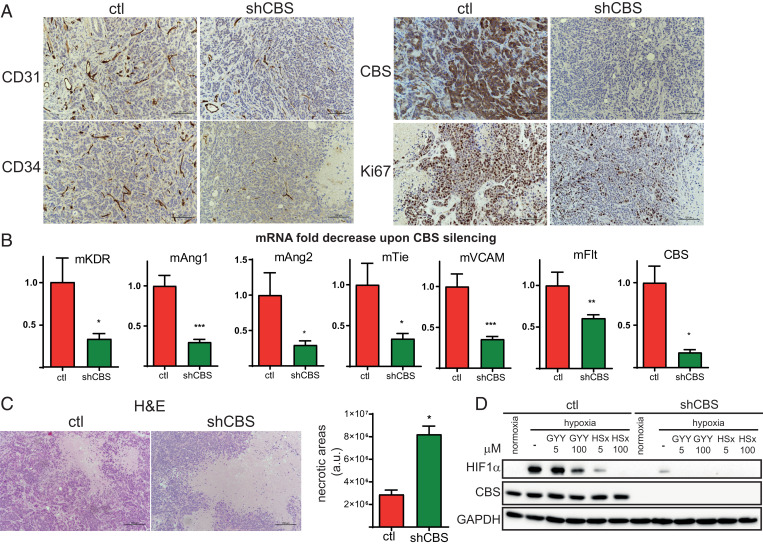
Fig. 3.
CBS plays an important role in cell proliferation, angiogenesis, intratumoral cancer cell necrosis, and the hypoxic response in BLBC. (A) Immunohistochemistry of HCC1143 control and shCBS xenograft tissues. Proliferation of tumor cells were examined with Ki67 nuclear antigen staining. The formation of intratumoral blood vessels was visualized using CD31 and CD34 markers. CBS protein presence and silencing were confirmed by CBS staining. (B) Impaired angiogenesis in shCBS HCC1143 xenograft tumors were further confirmed at the mRNA level by RT-qPCR; kinase insert domain protein receptor (KDR), angiopoetin (Ang) 1 and 2, tyrosine kinase with immunoglobulin-like and EGF-like domains 1 (Tie), FMS-like tyrosine kinase 1 (Flt), vascular cell adhesion molecule 1 (VCAM), and CBS mRNA changes were compared to controls and normalized to actin. (C) Histological assessment of intratumoral necrosis in control and shCBS HCC1143 xenografts. (Scale bars: 200 µm.) Necrotic areas were evaluated by the ImageJ software. Representative images are shown. (D) In vitro analyses of HIF1-α–mediated hypoxia response in control and shCBS Cal51 cells. Cells were pretreated with 5 and 100 µM sulfide donor GYY4137 (GYY) for 90 min or with 5 and 100 µM polysulfide (HSx−) for 30 min where indicated. After 6 h of hypoxia or normoxia, cells were collected, and HIF1-α activations were assessed by Western blot analysis. Experiments were repeated three times, and representative images are shown. *P < 0.05, **P < 0.01, and ***P < 0.001 show significant differences in shCBS compared to control samples in B and C.