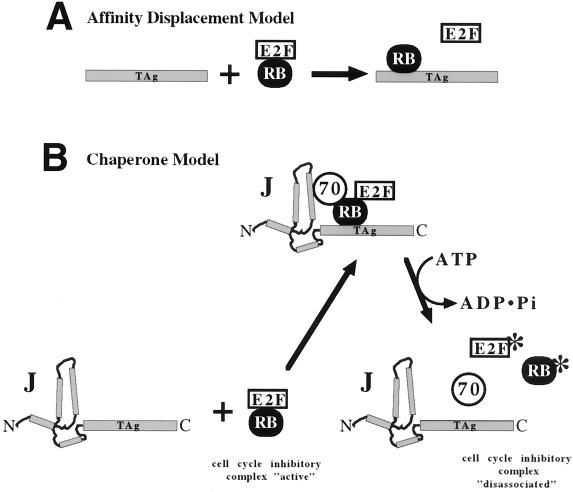
FIG. 6.
Models for T-antigen-induced dissociation of pRb-E2F family complexes. Disruption of growth-inhibitory complexes (labeled Rb/E2F) drives cells to enter the cycle. (A) Affinity displacement model. T antigen (TAg) binds to pRb with a higher affinity for E2F than pRb's affinity for E2F, thus sequestering pRb from E2F. (B) Chaperone model. T antigen, with cochaperone Hsc70 and ATP hydrolysis, elicits a conformational change in the pRb-E2F complex or T antigen, thereby rearranging the complex and decreasing the amount of pRb associated with E2F. Note that T antigen does not remain associated with pRb. Some other modification (denoted with an asterisk) of pRb or E2F may be required to keep proteins from reassociating. To underscore the role of the J domain, it is depicted as four interacting rods which represent the four alpha helices of the J domain (21) at the amino terminus (N) of T antigen.