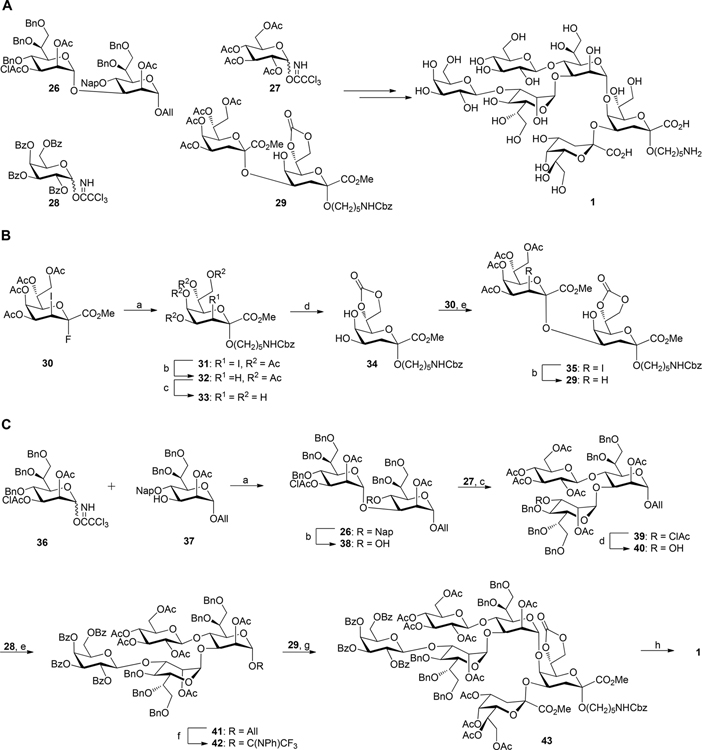
Scheme 1. (A) Chemical Synthesis of Inner Core Hexasaccharide 1 and (B) Synthesis of Kdo2 Glycosyl Acceptor 29a.
aReagents and conditions: (a) HO(CH2)5NHCbz, BF3·Et2O, CH2Cl2, 3 Å molecular sieves, 0 °C to rt, 2.5 h, 75%; (b) lauroyl peroxide, ClCH2CH2Cl/cyclohexane 1:7, reflux at 90 °C, 2 h, 83% for 32, 87% for 29; (c) NaOCH3/CH3OH, rt, 4 h, 92%; (d) diphosgene, sym-collidine, THF, −25 °C, 30 min, 76%; (e) BF3·Et2O, CH2Cl2, 3 Å molecular sieves, 0 to 15 °C, 2 h, 80%. (C) Assembly of hexasaccharide 1, reagents and conditions: (a) TMSOTf, CH2Cl2, 4 Å molecular sieves, −20 to −10 °C, 30 min, 89%; (b) DDQ, CH2Cl2/PBS buffer (100 mM, pH 7.4) 10:1, rt, 1.5 h, 78%; (c) TMSOTf, CH2Cl2, 4 Å molecular sieves, 0 to 5 °C, 70 min, 80%; (d) thiourea, sym-collidine, CH2Cl2/CH3OH 2:3, 70 °C, 24 h, 85%; (e) TMSOTf, CH2Cl2, 4 Å molecular sieves, −10 to 0 °C, 60 min, 90%; (f) (i) PdCl2, CH2Cl2/MeOH 1:5, rt, 2.5 h, 86%; (ii) CF3C(NPh)Cl, Cs2CO3, CH2Cl2, rt, 4 h, 85%; (g) TMSOTf, CH2Cl2, 5 Å molecular sieves, rt, 2 h, 40%; (h) (i) 1 M NaOH(aq)/CH3OH/dioxane 1:1:3, rt, 16 h, (ii) H2, Pd(OH)2/C, t-BuOH/H2O/HOAc 3:2:0.02, rt, 18 h, 42% over 2 steps.