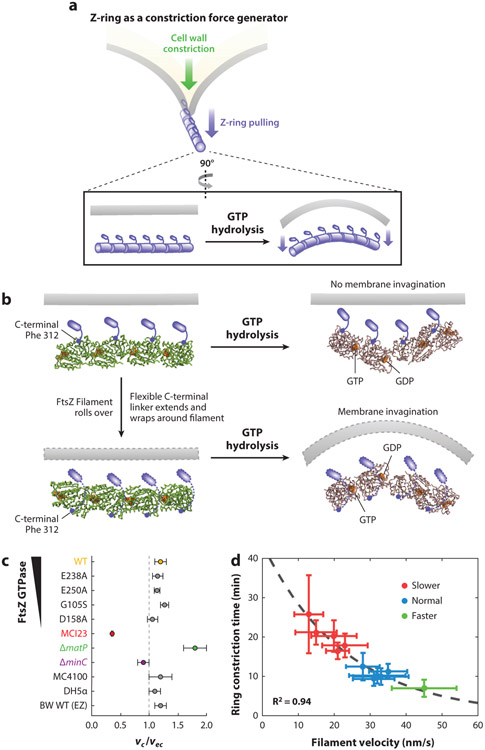
Figure 8.
(a) Schematics of the Z-ring functioning as a constriction force generator through GTP hydrolysis-induced filament bending. (b) A recent crystallographic study (114) shows that GTP hydrolysis causes the FtsZ protofilament to bend, but the bending is toward the membrane (top) if the C-terminal tail of FtsZ (small blue rod) is directly attached to the membrane, which is opposite the direction of membrane invagination. To accommodate the bending conformation, the flexible linker between the globular domain and C-terminal tail of FtsZ has to wrap around the filament to attach to the membrane (dotted outlines, bottom). Note that the models shown are for visualization purposes only and are not based on the molecular dynamics simulations done in Reference 114. (c) Growth condition–normalized septum closure rate measurements made in wild-type (WT) BW25113 Escherichia coli cells in minimal media compared with different background strains including mutants affecting FtsZ’s GTPase activity (E238A, E250A, G105S and D158A), FtsI’s activity (MC123), chromosome segregation (ΔmatP), and Z-ring stability (ΔminC), in addition to different WT strain backgrounds (MC4100, DH5α) and WT BW25113 cells growing in rich defined growth medium (EZ). The FtsI mutant constricts significantly slower than WT cells, ΔmatP constricts faster, and all other perturbations of the Z-ring did not produce significant changes in septum closure rates (40, 239) (d) Constriction time measured in a series of Bacillus subtilis strains showed a high correlation with Z-ring treadmilling speed (20).