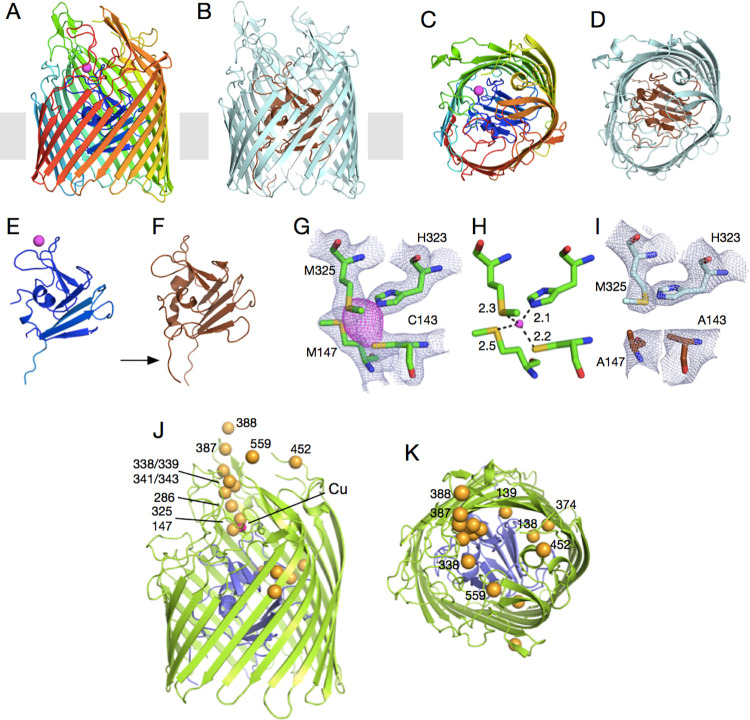
Fig 1. OprC is a TBDT that binds ionic copper via an unprecedented CxxxM-HxM binding site.
Cartoon representation of (A, C) Cu-loaded OprC and (B, D) Cu-free OprC (OprCAA). (A, B) are viewed from the OM plane, whereas the views for (C, D) are from the outside of the cell. The N-terminal plug domain is shown separately for both forms (E, F). For Cu-OprC, structures are shown in rainbow from N-terminus (blue) to C-terminus (red); copper is represented as a magenta sphere. Apo-OprC is coloured light cyan, with the plug rendered brown. The arrow in (F) highlights the visibility of the Ton box in apo-OprC. (G) Stick models of copper-coordinating residues Cys143, Met147, Met325, and His323. Electron density in grey mesh (2Fo-Fc map contoured at 2.0σ, carve = 2.0) is shown for the binding site residues C/M-H/M and the copper atom (anomalous difference map shown in magenta, contoured at 3.0σ, carve = 2.25). (H) Distances between coordinating residues and metal show that copper is coordinated via 1 thiolate (from Cys), 2 thioethers (from Met), and 1 imidazole nitrogen from His. (I) Mutation of binding site residues Cys143 and Met147 to alanines in OprCAA abolishes copper binding (2Fo-Fc map contoured at 2.0σ, carve = 2.0). (J, K) OM plane (J) and extracellular views (K) showing the thioether atoms of all methionine residues in Cu-OprC as yellow spheres. The copper atom, only visible in (J), is shown as a magenta sphere. OM, outer membrane; TBDT, TonB-dependent transporter.