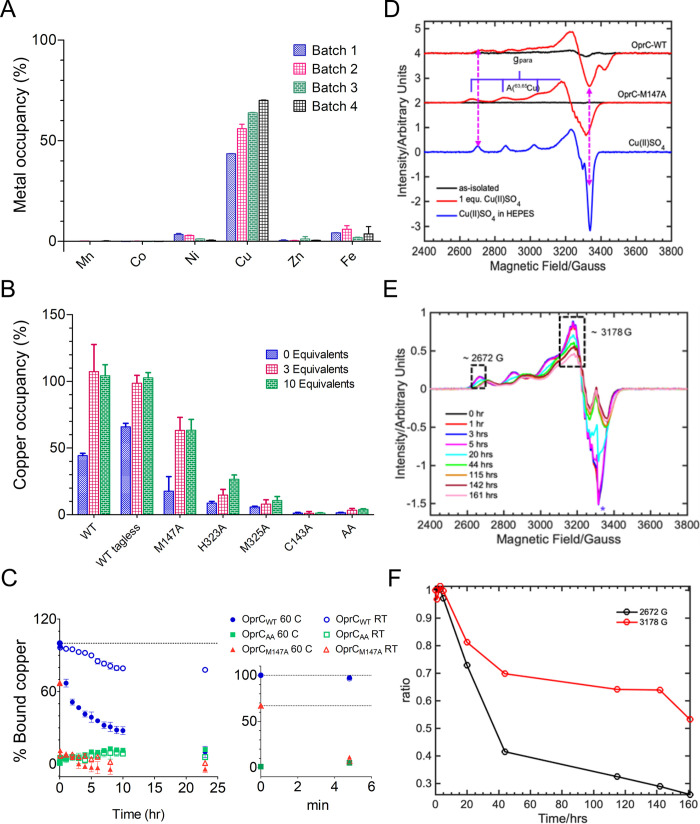
Fig 2. OprC binds 1 equivalent copper near-irreversibly.
(A) Metal occupancy of LB-purified WT OprC by ICP-MS shows specific binding only to copper. Each colour indicates individual batches of protein purified from rich media. (B) Copper content of WT OprC and binding site mutant proteins before (blue) and after aerobic incubation with either 3 (pink) or 10 (green) equivalents Cu(II) for 30 min followed by analytical SEC. All proteins contain a N-terminal His7 tag except where stated. (C) Copper is kinetically trapped in OprC. Time course of copper extraction experiments showing % bound copper for OprCWT (blue), OprCAA (green), and OprC M147A (red), at RT (open symbols) and 60°C (filled symbols). The inset shows % bound copper in the first few minutes after starting the experiment. OprCAA served as a control. Dotted lines indicate initial occupancies of OprCWT and M147A. (D) Comparison of the cw-EPR spectra of OprCWT and OprCM147A mutant before (black traces) and after (red traces) addition of 1 equivalent Cu(II) solution. The blue trace shows the EPR spectrum of the Cu(II)SO4 in Hepes buffer. All EPR spectra have been background subtracted. The double-headed magenta dotted arrows show the difference in the observed g and A tensor of OprC variants. The blue goal posts indicate the 63,65Cu-hyperfine splitting along the parallel region. Note that the starting copper equivalencies for these proteins were 0.6 (OprCWT) and 0.1 (OprCM147A), respectively. (E) EPR time course for OprCM147A after addition of 1 equivalent Cu (II). (F) Relative intensities of EPR signals at approximately 2,672 G and 3,178 G (black dotted rectangular boxes in the top panel) plotted as a function of time. Values shown are averages from 3 independent time courses. Underlying data for this figure can be found in S1 Data. EPR, electron paramagnetic resonance; ICP-MS, inductively coupled plasma mass spectrometry; RT, room temperature; SEC, size exclusion chromatography; WT, wild-type.