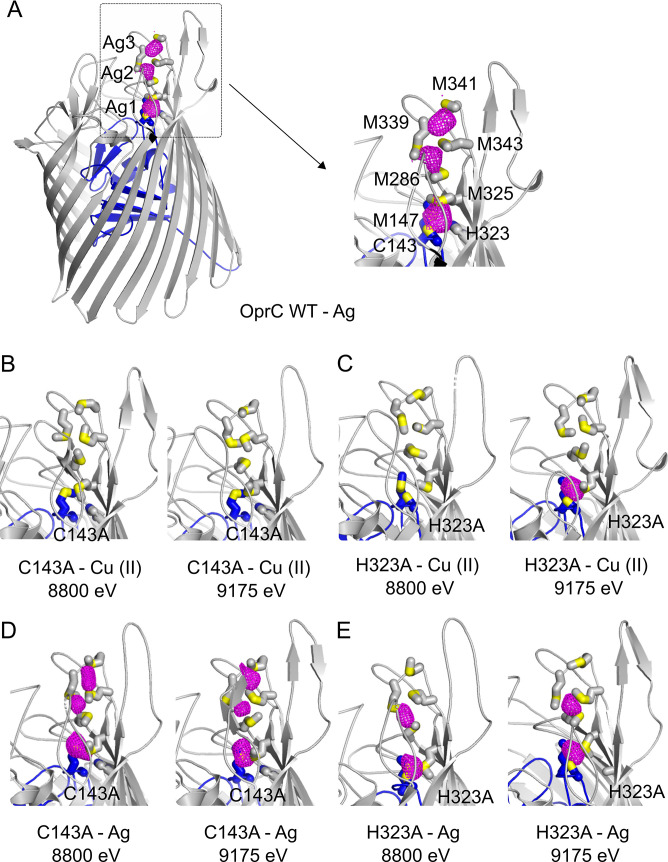
Fig 3. OprC binds Ag at the high-affinity binding site and the methionine track.
(A-E) Anomalous difference maps of (A) OprCWT, (B, D) C143A, and (C, E) H323A variants crystallised in the presence of (A, D, E) Ag or (B, C) Cu(II) and collected at different energies. The inset to (A) shows a close-up of the anomalous difference peaks (magenta) near the principal binding site in OprCWT, with binding residues labelled and represented as stick models. Sulphurs are coloured yellow. For clarity, the metal used in cocrystallisation and the energy used for data collection are shown underneath each panel. The OprC plug domain is coloured blue. WT, wild-type.