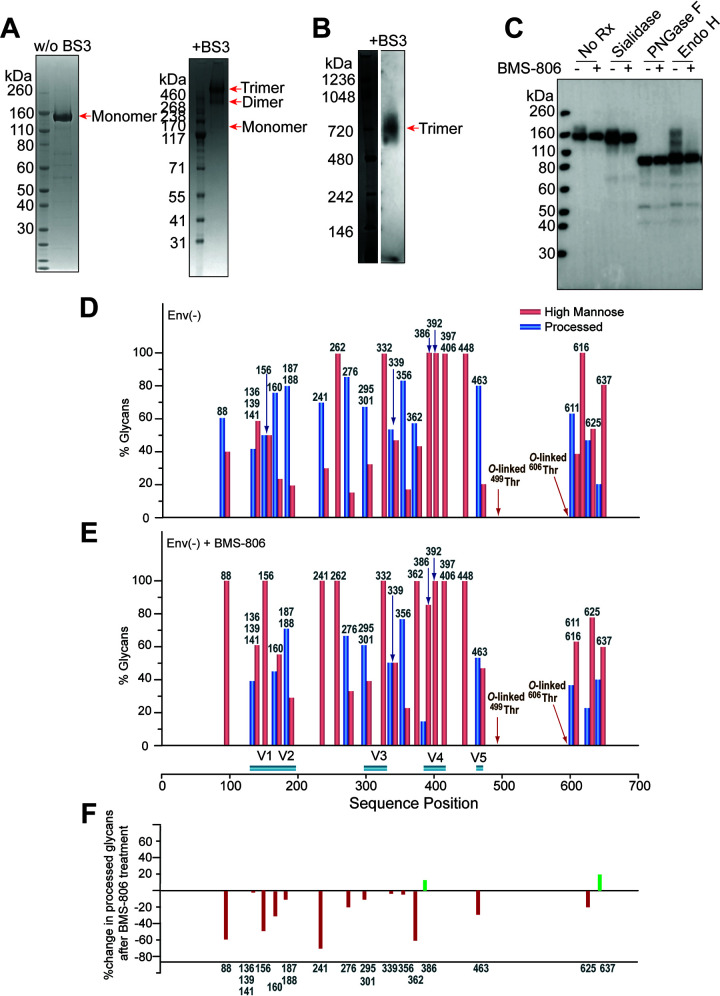
FIG 2.
Characterization of the full-length HIV-1JR-FL Env(−) glycoprotein in CHO cell lysates and in detergent-solubilized purified forms. (A) Purified HIV-1JR-FL Env(−) without and with cross-linking by BS3 was run on a NUPAGE 4-to-12% BT gel stained by Coomassie blue. (B) Purified HIV-1JR-FL Env(−) cross-linked by BS3 was run on a native PAGE 4-to-16% Bis-Tris gel and subjected to Western blotting with an HRP-conjugated anti-HIV-1 gp120 antibody. (C to E) To evaluate the effect of BMS-806 on the glycosylation of the synthesized Env(−) glycoprotein, BMS-806 (10 μM) was added to the CHO cells at the time of doxycycline induction. (C) The effect of BMS-806 on HIV-1JR-FL Env(−) glycosylation was evaluated by Western blotting after digestion with glycosidases (sialidase, peptide-N-glycosidase F [PNGase F], and endoglycosidase H [Endo H]). The purified HIV-1JR-FL Env(−) glycoproteins were digested with the indicated glycosidase, run on a NUPAGE 4-to-12% Bis-Tris gel, and subjected to Western blotting with a goat anti-HIV-1 gp120 antiserum. The results shown are representative of those obtained in three independent experiments. Note that BMS-806 treatment decreases Env(−) heterogeneity by reducing the levels of sialidase-sensitive and Endo H-resistant glycoforms. (D and E) The bar graphs show the glycan profiles at each glycosylation site of HIV-1JR-FL Env(−) purified from untreated CHO cells (D) or CHO cells treated with 10 μM BMS-806 (E), as determined by mass spectrometry. The glycan composition (in percent) was broadly characterized as high-mannose (red bars) or processed (complex plus hybrid) glycans (blue bars). (F) The results in panels D and E were used to calculate the change in the percentage of processed glycans after BMS-806 treatment, which is shown for each N-linked glycosylation site.