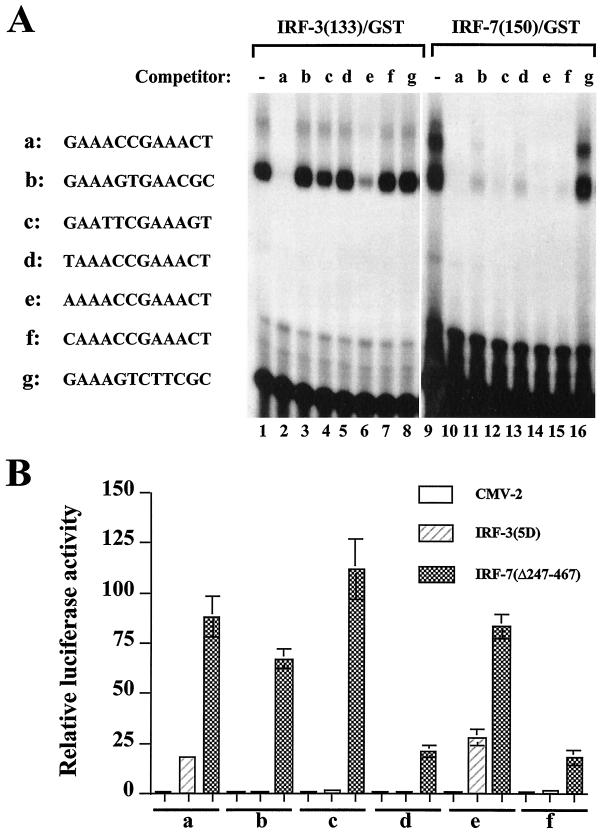
FIG. 8.
Characterization of selected binding sites. (A) An EMSA was performed with 20 ng of recombinant IRF-3–GST (lanes 1 to 8) or IRF-7–GST (lanes 9 to 16), 32P-labeled oligonucleotide a (5′-GAAACCGAAACTGAAACCGAAACT-3′), and a 1,000-fold molar excess of competitor DNA. Selected binding sites (two copies, indicated beside the gel as a to g) were used as competitors. (B) Activation of selected promoters by IRF-3 and IRF-7. 293 cells were transfected with the pRLTK control plasmid, reporter constructs containing the minimum TK-luciferase promoter and two copies of selected binding sites (designated a to f), and the active forms of IRF-3(5D) and IRF-7(Δ247–467) expression plasmids, and luciferase activity was analyzed at 24 h posttransfection. Relative luciferase activity was measured as fold activation (relative to the basal level for the reporter gene in the presence of the pFlag-CMV-2 vector after normalization to cotransfected relative light unit activity); the values represent the average of three experiments performed in duplicate, with variability of 10 to 25%.