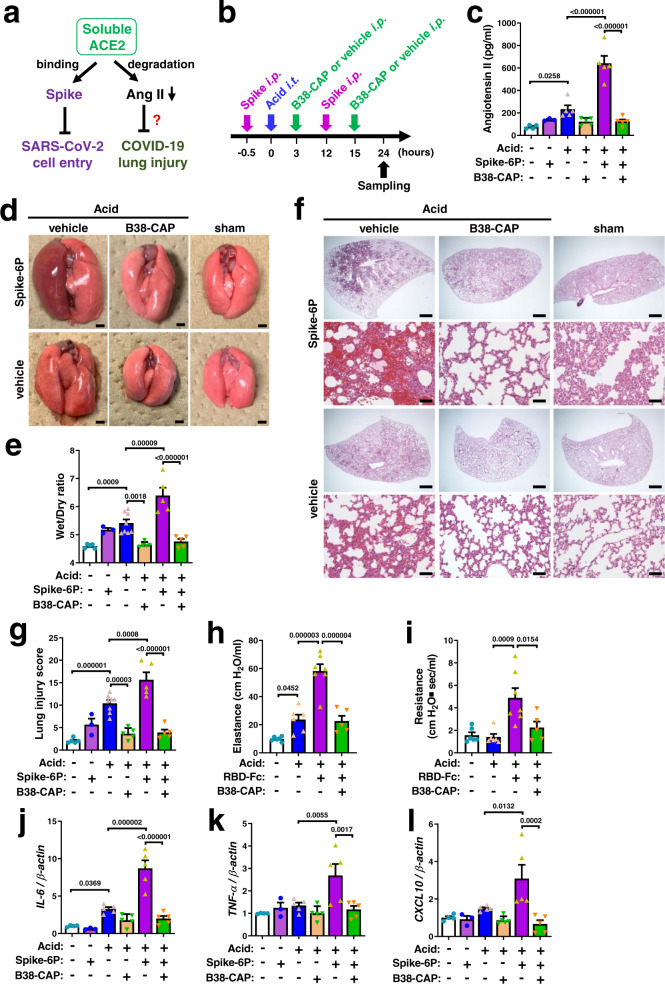
Fig. 3. Suppression of SARS-CoV-2 Spike-induced lung injury by B38-CAP.
a Effects of soluble ACE2 in SARS-CoV-2 infection and lung injury. b Experimental protocol of B38-CAP treatment for hamsters with acid and Spike-induced lung injury. Spike (trimeric Spike-6P protein (3.7 nmol/kg) or RBD-Fc (11 nmol/kg)) or control with or without B38-CAP (2 mg/kg) were intraperitoneally injected (i.p.), and acid (0.1 N HCl, 100 μl per body) was intratracheally instilled (i.t.) under anesthesia. c Plasma Ang II measurements at 24 h after acid instillation (n = 4 hamsters for sham + vehicle, n = 3 for sham + Spike-6P, n = 5 each for other experimental groups). d Representative photograph of hamster lungs. Bars indicate 2 mm. e Wet to dry weight ratios of lungs at 24 h after acid instillation (n = 3 hamsters for sham + Spike-6P, n = 6 for Acid + vehicle, n = 5 each for other experimental groups). f, g Lung histopathology. Tissue samples were harvested at 24 h after acid instillation. Representative images are shown (f). Bars indicate 1 mm (upper) and 100 μm (bottom) for each treatment. Lung injury score measurements (g) (the same experimental cohort as e). h, i Lung function measurements. Elastance (h) and resistance (i) were measured at 17 h after acid instillation (n = 6 hamsters each for Sham + control-Fc + vehicle and Acid + control-Fc + vehicle, n = 7 for Acid + RBD-Fc + vehicle, n = 5 for Acid + RBD-Fc + B38-CAP). j–l qRT-PCR analysis of pro-inflammatory cytokine expression in the lungs of hamsters; mRNA levels of IL-6 (j), TNF-α (k), and CXCL10 (l) normalized with β-actin (the same experimental cohort as c). All values are means ± SEM. One-way ANOVA with Sidak’s multiple comparisons test. Numbers above square brackets show significant P values. Independent experiments were performed two times (c–l), and consistent results were obtained.