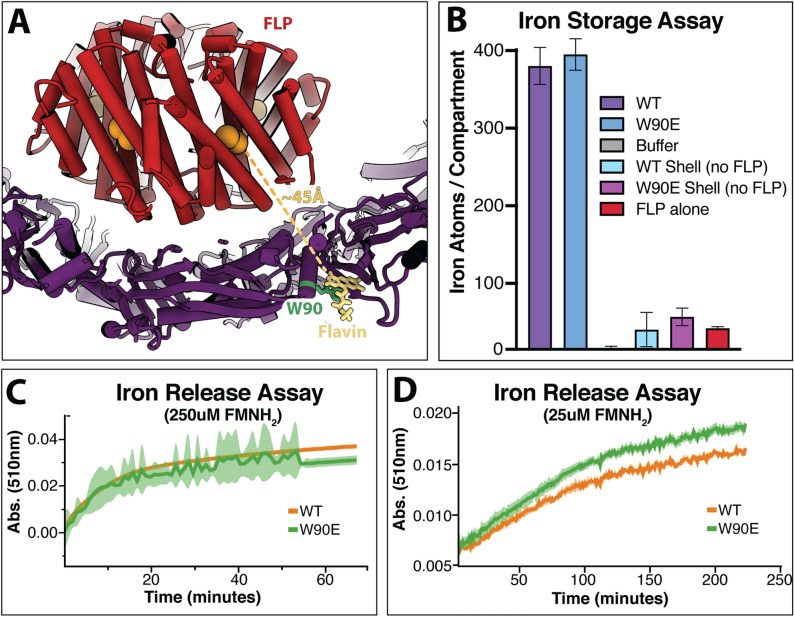
Figure 5.
Biochemical data regarding the role of flavin in iron mineralization and iron mobilization. (A) Depiction of the distance between the FLP active site and the FMN molecule bound to the shell, with FLP in red, the active site in orange, the shell in purple, the bound FMN in yellow, and the interacting W90 residue in green. (B) shows the ferroxidase iron storage activity of various encapsulin constructs. ‘WT’ is the unmodified encapsulin with FLP cargo protein; ‘W90E’ also contains the FLP cargo protein but also represents the W90E mutation to the shell; ‘WT Shell’ does not contain the FLP cargo; ‘W90E Shell’ is also lacking the FLP and contains the W90E mutation; and ‘FLP alone’ represents the FLP cargo protein free in solution that was purified without the encapsulin shell. The opposite activity, the release of iron, is shown in (C) at 250uM FMNH2 and (D) at 25 uM FMNH2. FLP-loaded encapsulin constructs with a ferric oxide core were used for the experiments in (C) and (D), with the green curve representing W90E-encapsulin without flavin, and the orange trace is WT FLP-encapsulin. (C) and (D) lines represent n = 3 average, with the envelope showing standard deviation.