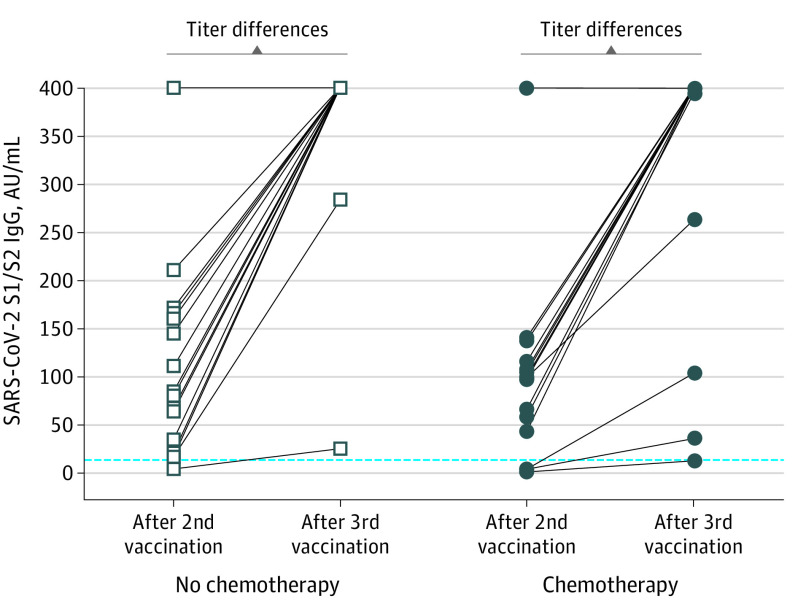
Figure. Serologic Response to a Third Dose of the SARS-CoV-2 BNT162b2 mRNA Vaccine in Patients With Cancer Undergoing Active Treatment.
Comparison between antibody levels after the second dose and the third booster dose of BNT162b2 (Pfizer-BioNTech) in patients treated with chemotherapy and nonchemotherapy regimens. Measurements less than 12 AU/mL are considered negative, 12 to 19 AU/mL are equivocal, and greater than 19 are positive (dashed line). Levels above 60 AU/mL were shown to be protective. The upper antibody titer limit was capped at more than 400 AU/mL; thus, the titer differences may be higher than those recorded. The titer increment in both groups is statistically significant at P < .001.