Figure 1.
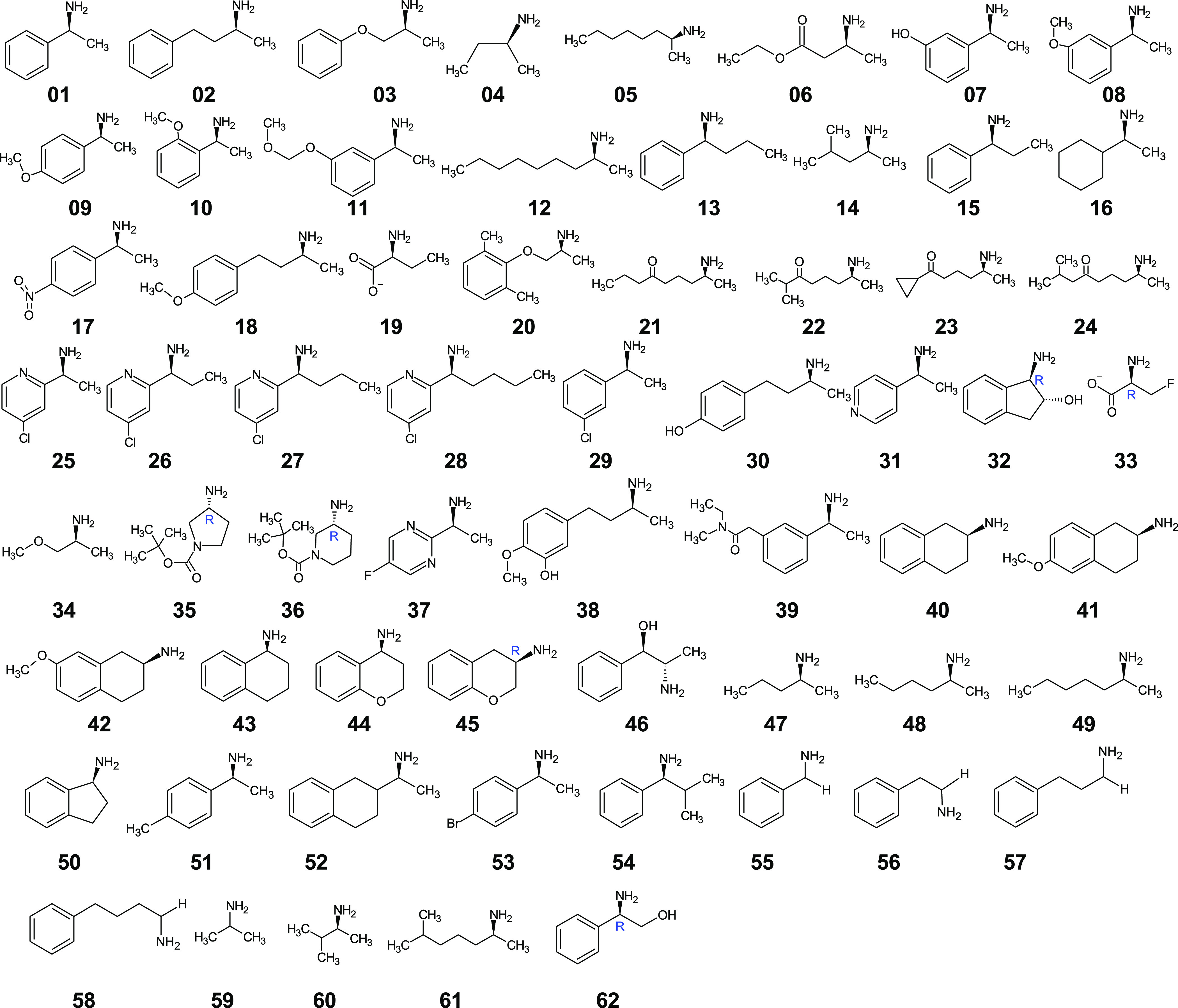
Compound dataset used in this study as benchmark to test the reliability of the developed protocol. The dataset was gathered from the existing literature, and sources are cited in Table 1. The enantiomeric excess for the asymmetric synthesis reactions used to obtain amines 01–49 from their respective ketones has been reported. Compounds 50–62 were only used for substrate-scope predictions because they are nonchiral or the experimental ee% for asymmetric synthesis was not found in the literature. All structures represent the preferred enantiomer of Vf-TA and are drawn with the bulkiest substituent to the left, i.e., in the position corresponding to the phenyl group of the (S)-enantiomer of 01. Following CIP rules, all compounds are (S)-amines, except 32, 33, 35, 36, 45, and 62 which formally are (R)-enantiomers.
