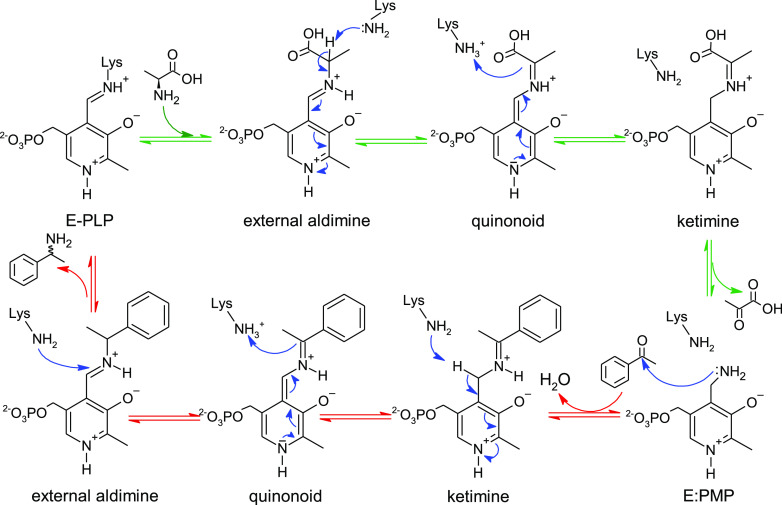
Scheme 1. Transamination Reaction Mechanism.
The fully reversible reaction consists of two half-transamination reactions.1 In the first half reaction (green arrows), l-alanine is converted into pyruvate, resulting in the generation of the E/PMP intermediate (PMP is pyridoxamine-5′-phosphate). The second half (red arrows) consists of substrate entry and formation of the Michaelis complex with E:PMP. A nucleophilic attack on the carbonyl carbon of the substrate (acetophenone shown as an example) by the amino group of PMP leads to the formation of the ketimine intermediate. A further rearrangement involves the catalytic lysine (Lys285), acting first as a base and later as an acid, to form the quinonoid and external aldimine intermediates, respectively. Finally, Lys285 performs a nucleophilic attack on the iminium carbon of the external aldimine, resulting in the formation of amine (1-phenylethylamine) and an internal aldimine (E-PLP).