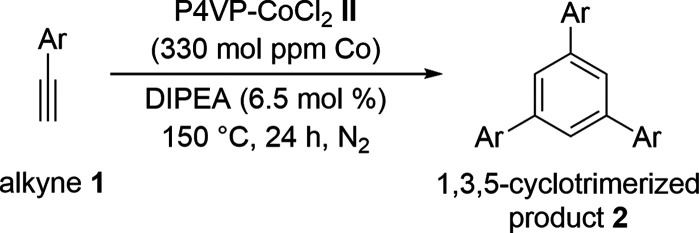
Table 2. Substrate Scope for Cyclotrimerizationa.
| entry | substrate 1 (Ar) | yield of 2 (%) |
|---|---|---|
| 1 | Ph, 1a | 68 |
| 2 | 4-MeC6H4, 1b | 84 |
| 3 | 3-MeC6H4, 1c | 77 |
| 4 | 2-MeC6H4, 1d | 67 |
| 5 | 4-MeOC6H4, 1e | 86 |
| 6 | 4-BuC6H4, 1f | 79 |
| 7 | 4-BrC6H4, 1g | 66b |
| 8 | 4-ClC6H4, 1h | 61b |
| 9 | 4-FC6H4, 1i | 53 |
| 10 | 4-CF3C6H4, 1j | 42 |
| 11 | 4-EtO2CC6H4, 1k | 46 |
| 12 | 4-MeCOC6H4, 1l | 39b |
| 13 | 4-HOC6H4, 1m | 71b |
| 14 | 4-PhC6H4, 1n | 49b |
| 15 | 2-PhC6H4, 1o | 42b |
| 16 | 3-Py, 1p | 0b |
| 17 | 1-naphthyl, 1q | 41 |
| 18 | 1-octyne (1r) | 0 |
| 19 | (triisopropylsilyl)acetylene (1s) | 0 |
| 20 | 1,2-diphenylethyne (1t) | 0b |
Reaction conditions: 1 (1 mol equiv., 5 mmol), P4VP-CoCl2 (0.033 mol %, 330 mol ppm of Co), DIPEA (6.5 mol %), 150 °C, 24 h, N2 atmosphere. Isolated yield.
One-half a milliliter of xylene was added.